Non-Response to Cardiac Resynchronization Therapy: How Do We Turn the Tide?
Cardiac resynchronization therapy (CRT) has been an important adjunctive therapy for congestive heart failure patients (CHF) with prolonged QRS and reduced left ventricular (LV) systolic function who remain symptomatic despite optimized 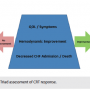 pharmacologic therapy.1-4 However, multicenter randomized control trials have demonstrated a CRT “non-responder” rate ranging from 32-43% at six months.2,3 Much of this range also varies based upon the definition of responders or non-responders. In addition, the complexity of CHF treatments and the degree of symptomatology all play roles in how we manage our patients. In this article, we will discuss the steps needed for an effective treatment strategy for CRT non-responders.
pharmacologic therapy.1-4 However, multicenter randomized control trials have demonstrated a CRT “non-responder” rate ranging from 32-43% at six months.2,3 Much of this range also varies based upon the definition of responders or non-responders. In addition, the complexity of CHF treatments and the degree of symptomatology all play roles in how we manage our patients. In this article, we will discuss the steps needed for an effective treatment strategy for CRT non-responders.
The first step in tackling non-responders is to establish a standardized institutional definition of non-response. This collaborative process should include the resident heart failure specialist, electrophysiologist, cardiologist, and all of the members involved in the patient’s treatment team. We use a triad approach for the identification and assessment of a successful response to CRT, which begins with decreasing CHF admissions and includes the primary goal of decreasing mortality. (Figure 1) In addition, improvements in patient symptoms and quality of life are crucial in defining the responder status. Finally, some method of hemodynamic assessment, or surrogate of hemodynamic assessment, should be employed as the third component of identifying responder status. At our institution, if two of these three components are not improved with CRT, the patient is assigned a non-responder status and our non-responder protocol is initiated.
As a first step, a full evaluation consisting of medication review, BNP levels, and physical examination and work-up begin the process. Once a confirmation of non-response is verified with full examination, the IPAQ-DW (Imaging, Placement, Arrhythmia, Quick to optimize, Don’t Wait) protocol begins. (Figure 2)
IMAGING
First and foremost, a 12-lead ECG should be assessed to ensure the QRS morphology indicates biventricular pacing is being delivered. This should include an initial positive R wave in lead V1 as proposed by Sweeney et al.5 In addition, the 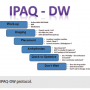 P wave should be assessed to ensure LV stimulation is not initiated prior to the completion of the P wave, which may indicate that the left atrium is still contracting when the LV is activated. This “left-sided pacemaker syndrome” is frequently a culprit of non-response due to aggressive AV delay programming to ensure delivery of CRT. The next step should be sending the patient for PA and lateral chest x-ray to confirm the lead position relative to implant, to rule out macro LV lead dislodgment. An echocardiographic dyssynchrony study, which has been shown to have positive contributions to CRT outcomes, may then be utilized to compare dyssynchrony from the pre-operative evaluation.6-10
P wave should be assessed to ensure LV stimulation is not initiated prior to the completion of the P wave, which may indicate that the left atrium is still contracting when the LV is activated. This “left-sided pacemaker syndrome” is frequently a culprit of non-response due to aggressive AV delay programming to ensure delivery of CRT. The next step should be sending the patient for PA and lateral chest x-ray to confirm the lead position relative to implant, to rule out macro LV lead dislodgment. An echocardiographic dyssynchrony study, which has been shown to have positive contributions to CRT outcomes, may then be utilized to compare dyssynchrony from the pre-operative evaluation.6-10
PLACEMENT
This step is an important second stage to the initial imaging evaluation. The LV lead should not only be confirmed as having the same location compared to implant, but also be compared with the operative note and lab fluoroscopy and/or coronary sinus venograms to verify appropriate lead placement at the time of implant. This second look allows considerations in programming, such as other LV pacing vectors which may be utilized; some patients respond preferentially to more basal or apical LV pacing.11 Another consideration to LV vector programming is the utilization of an LV cathode from a site with the maximal separation to the right ventricular pacing electrode, either spatially or electrically based upon the paced or sensed electrical delay, which has also shown benefit in some patients.12-15
ARRHYTHMIAS
One additional component of CRT response that is sometimes overlooked is the association of arrhythmia burden. Either new-onset atrial arrhythmias or a progression of atrial arrhythmias may lead to subtle changes in the amount and efficiency of CRT delivered to a patient. Another important component in the assessment of non-responders is the percent of biventricular pacing. Sometimes, the percent of ventricular pacing indicated by a CRT device may not equate to the delivery of true biventricular pacing. This is because of the possibility that the CRT device is delivering biventricular pacing into a conducted or partially conducted activation, which the device logs as pacing. In this scenario, the LV activation may be entirely different and suboptimal compared to that from de novo biventricular pacing. This phenomenon is frequently encountered in patients with atrial fibrillation. Additionally, an increase in ventricular arrhythmias indicated by PVC burden may also affect optimal CRT in similar form to atrial arrhythmias.
QUICK TO OPTIMIZE
Similarly in the effect of LV lead placement, optimization of AV and VV delays proves to be an important component of effective CRT. Optimization may range from automated electrogram-based alterations in timing of the AV delay or VV delay settings, to utilizing echocardiographic optimization of flow. The utility of electrogram-based algorithms has been demonstrated in clinical trials and is an easy first step in optimization.16-18 The gold standard continues to be assessment of blood flow-based changes that vary with these delays, which can be altered with a programmer at the time of echocardiography.4,19-21 This method allows visualization of proper AV delay settings by ensuring there is little to no truncation of the A wave and no E/A fusion is occurring. In addition, the VV delay setting may be optimized to ensure optimal VTI, LVESV, or reduced dyssynchrony.
DON’T WAIT (DW)
One of the most important aspects of the CRT non-response protocol is the final step: “don’t wait.” Although it comes at the end, this step impacts every step along the way in treating non-responders. This step should even apply to patients prior to confirming their non-response, because preventing patient disease progression is paramount. After CRT is initiated, if a degradation in symptoms, health, or quality of life occurs, this can accelerate quickly in these patients. Assessment and action in the optimization of patients should be done at each healthcare interaction, since these patients are generally observed only every three to four months in routine CRT follow-up.
CONCLUSION
The impact of CRT non-response not only poses a significant burden on the clinical course and outcome of patients, but the economic viability of healthcare institutions and our healthcare system in general. CHF accounts for over 50% of Medicare reimbursement for patients over 65, with heart failure readmission accounting for a majority of that cost.22-24 The average CHF admission cost ranges with the degree of comorbid conditions and major complications from $8,452 to $10,000 in the U.S., translating to a cost of approximately $8 billion annually to Medicare, and an estimated $20-56 billion of annual direct expenditure of all U.S. healthcare dollars.25-28 Data to evaluate the exact cost of CRT non-response is very limited; however, following appropriate treatment guidelines, one would conclude that an effective treatment strategy would help not only the bottom line for patients, but also any healthcare systems’ bottom line.
Disclosures: Dr. Corbisiero has no conflict of interest to report; David Muller is an employee of St. Jude Medical.
References
- Hunt SA; American College of Cardiology; American Heart Association Task Force on Practice Guidelines (Writing Committee to Update the 2001 Guidelines for the Evaluation and Management of Heart Failure). ACC/AHA 2005 Guideline Update: Diagnosis and Management of Chronic Heart Failure in the Adult. J Am Coll Cardiol. 2005;46(6):e1-82.
- Abraham WT, Fisher WG, Smith AL, et al. Cardiac Resynchronization In Chronic Heart Failure. N Engl J Med. 2002;346(24):1845-1853.
- Bristow MR, Saxon LA, Boehmer J, et al. Cardiac-Resynchronization Therapy with or without an Implantable Defibrillator in Advanced Chronic Heart Failure. N Engl J Med. 2004;350(21):2140-2158.
- Jansen AH, Bracke FA, van Dantzig JM, et al. Correlation of echo-Doppler optimization of atrioventricular delay in cardiac resynchronization therapy with invasive hemodynamics in patients with heart failure secondary to ischemic or idiopathic dilated cardiomyopathy. Am J Cardiol. 2006;97:552-557.
- Sweeney MO, van Bommel RJ, Schalij MJ, Borleffs CJ, Hellkamp AS, Bax JJ. Analysis of Ventricular Activation Using Surface Electrocardiography to Predict Left Ventricular Reverse Volumetric Remodeling During Cardiac Resynchronization Therapy. Circulation. 2010;121:626-634.
- Bax JJ, Bleeker GB, Marwick TH, et al. Left ventricular dyssynchrony predicts response and prognosis after cardiac resynchronization therapy. J Am Coll Cardiol. 2004;44:1834-1840.
- Gorcsan J 3rd, Kanzaki H, Bazaz R, Dohi K, Schwartzman D. Usefulness of echocardiographic tissue synchronization imaging to predict acute response to cardiac resynchronization therapy. Am J Cardiol. 2004;93:1178-1181.
- Yu CM, Chau E, Sanderson JE, et al. Tissue Doppler echocardiographic evidence of reverse remodeling and improved synchronicity by simultaneously delaying regional contraction after biventricular pacing therapy in heart failure. Circulation. 2002;105:438-445.
- Yu CM, Gorcsan J 3rd, Bleeker GB, et al. Usefulness of tissue Doppler velocity and strain dyssynchrony for predicting left ventricular reverse remodeling response after cardiac resynchronization therapy. Am J Cardiol. 2007;100:1263-1270.
- Gorcsan J 3rd, Abraham T, Agler DA, et al. Echocardiography for cardiac resynchronization therapy: recommendations for performance and reporting—a report from the American Society of Echocardiography Dyssynchrony Writing Group endorsed by the Heart Rhythm Society. J Am Soc Echocardiogr. 2008;21:191-213.
- Singh JP, Klein HU, Huang DT, et al. Left ventricular lead position and clinical outcome in the Multicenter Automatic Defibrillator Implantation Trial–Cardiac Resynchronization Therapy (MADIT-CRT) trial. Circulation. 2011;123:1159-1166.
- Gold MR, Birgersdotter-Green U, Singh JP, et al. Relationship between Ventricular and Electrical Delay and LV Remodeling. Eur Heart J. 2011;32:2516-2542.
- Polasek R, Kucera P, Nedbal P, et al. Local electrogram delay recorded from left ventricular lead at implant predicts response to cardiac resynchronization therapy. BMC Cardiovasc Disord. 2012;12:34.
- Heist EK, Fan D, Mela T, et al. Radiographic left ventricular-right ventricular interlead distance predicts the acute hemodynamic response to cardiac resynchronization therapy. Am J Cardiol. 2005;96:685-690.
- Pappone C, et al. Left ventricular pacing from a site of late electrical activation improves acute hemodynamic response to cardiac resynchronization therapy. 632 SAT-B-07. Abstract presented Oct 2012.
- Baker JH 2nd, McKenzie J 3rd, Beau S, et al. Acute evaluation of programmer-guided AV/PV and VV delay optimization comparing an IEGM method and echocardiogram for cardiac resynchronization therapy in heart failure patients and dual-chamber ICD implants. J Cardiovasc Electrophysiol. 2007;18(2):185-191.
- Ellenbogen KA, Gold MR, Meyer TE, et al. Primary results from the SmartDelay determined AV optimization: a comparison to other AV delay methods used in cardiac resynchronization therapy (SMART-AV) trial: a randomized trial comparing empirical, echocardiography-guided, and algorithmic atrioventricular delay programming in cardiac resynchronization therapy. Circulation. 2010;122:2660-2668.
- Martin D, Lemke B, Birnie D, et al. Investigation of a novel algorithm for synchronized left-ventricular pacing and ambulatory optimization of cardiac resynchronization therapy: results of the adaptive CRT trial. Heart Rhythm. 2012;9;(11):1807-1814.
- Sawhney NS, Waggoner AD, Garhwal S, Chawla MK, Osborn J, Faddis MN. Randomized prospective trial of atrioventricular delay programming for cardiac resynchronization therapy. Heart Rhythm. 2004;1:562-567.
- Van Gelder BM, Bracke FA, Meijer A, Lakerveld LJ, Pijls NH. Effect of optimizing the VV interval on left ventricular contractility in cardiac resynchronization therapy. Am J Cardiol. 2004;93:1500-1503.
- Vanderheyden M, De Backer T, Rivero-Ayerza M, et al. Tailored echocardiographic interventricular delay programming further optimizes left ventricular performance after cardiac resynchronization therapy. Heart Rhythm. 2005;2:1066-1072.
- Funk M, Krumholz H. Epidemiologic and economic impact of advanced heart failure. J Cardiovasc Nurs. 1996;10:1-10.
- Wolinsky FD, Smith DM, Stump TE, Overhage JM, Lubitz RM. The sequalae of hospitalization for congestive heart failure in older adults. J Am Geriatric Soc. 1997;45:558-563.
- Krumholz HM, Parent EM, Tu N, et al. Readmission after hospitalization for congestive heart failure among Medicare beneficiaries. Arch Intern Med. 1997;157:99-104.
- Fisher ES, Wennberg JE, Stuker TA, Sharp SM. Hospital readmissions rates for cohorts of Medicare beneficiaries in Boston and New Haven. N Engl J Med. 1994;331:989-995.
- Ghali JK, Cooper R, Ford E. Trends in hospitalization rates for heart failure in the United States, 1973-1986: evidence for increasing population prevalence. Arch Intern Med. 1990;150:769-673.
- Anderson GF, Steinberg EP. Hospital re-admissions in the Medicare population. N Engl J Med. 1984;311:1349-1353.
- Funk M, Krumholz H. Epidemiologic and economic impact of advanced heart failure. J Cardiovasc Nurs. 1996;10:1-10.











