Managing Heart Failure in a Complex Young Patient
Introduction
Heart failure (HF) is a complex and progressive disorder associated with reduced cardiac output, excessive peripheral vasoconstriction, renal dysfunction, and ventricular remodeling. HF is a public health crisis in the United States, with an estimated 6.6 million Americans affected. The 30-day readmission rates for patients with HF is nearly 25% among Medicare patients, and despite numerous initiatives to lower this number, readmissions have not decreased significantly.1 Three-year mortality rates for HF overall remain high at 50%.2
Obesity-related factors have been estimated to cause 11% of HF cases in men and 14% in women.3 The association between obesity and HF remains to be further elucidated, although it is speculated that the hemodynamic and myocardial changes induced by obesity could predispose the patient to cardiac dysfunction.3 Insulin resistance associated with obesity and diabetes have been implicated in the development of HF, but have not been fully explored.4 Other obesity comorbidities such as systemic hypertension, sleep apnea, and hypoventilation may further contribute to or exacerbate HF, resulting in a phenomenon described in the literature as obesity cardiomyopathy.5 Severe obesity appears to change hemodynamic performance and cause changes in cardiac morphology that can lead to left ventricular (LV) hypertrophy and left ventricular diastolic dysfunction.6
HF can be a challenging condition to treat in that patients typically require polypharmacy, and even subtle changes in the underlying cardiovascular condition may necessitate adjustment of the drug regimen. A multidisciplinary team approach to HF management has been advocated, but remains difficult to implement in real-world clinic practice. For optimal results, HF patients must be adherent to pharmacological treatment and communicate well with the clinical team. Factors that may adversely impact HF drug adherence have been identified in the literature and include increased severity of the disease, depression, cognitive decline, excessive daytime sleepiness, dialysis, the use of antiarrhythmic agents, increased dosing (twice daily or more frequently), living alone, and others. Higher education, previous HF hospitalization, good health literacy, and being married are factors associated with greater adherence.7
Case presentation
The patient was a 24-year-old male who presented to the clinic with cardiomyopathy, left bundle branch block, and a left ventricular ejection fraction of 12%. He was obese with a body mass index (BMI) of 55.2. Prior to presenting at the clinic, he had been on optimal pharmacological therapy for HF. Because his condition made him vulnerable to sudden cardiac arrest (SCA), he wore a wearable defibrillator. To address his HF, cardiac resynchronization therapy (CRT) was considered. In selecting an appropriate CRT device, it was considered optimal to reduce the hardware in this young patient, particularly since he did not have chronotropic incompetence or a need for atrial pacing. The Intica CRT-DX 7 HF-T QP (BIOTRONIK) system was selected. The Intica CRT-DX is a unique device that offers CRT with a two-lead system, but with three-channel functionality. The CRT-DX allows for atrial sensing and diagnostics without the need for implantation of an atrial lead. The benefits of having no atrial lead include less hardware in the body, avoiding atrial lead dislodgement or other lead-related complications, and a streamlined implantation procedure. The Intica CRT-DX relies on atrial dipoles located on the right ventricular (RV) lead that float in the atrial blood pool, allowing for atrial sensing and tracking (VDD pacing mode). If the patient develops chronotropic incompetence, an atrial lead can be added to the Intica CRT DX system without the need for a generator upgrade, and once an atrial lead is implanted, the device can be programmed to the DDD/DDD-CLS mode.
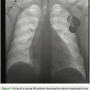
The patient was implanted with the Plexa® DX defibrillation lead (BIOTRONIK), which may be safely used with magnetic resonance imaging (MRI) under certain conditions (ProMRI, BIOTRONIK). The selected lead size was a Plexa DX 65/17. The latter number (17) refers to the dipole electrode spacing from the distal tip to the center of the dipole ring electrodes. A more frequently implanted lead is the Plexa DX 65/15 cm. The additional spacing required between the electrodes on the lead helped to accommodate the larger sized heart of this patient (Figure 1).
Due to the patient’s young age and comorbidities, MRI compatibility was an important consideration. The Intica CRT-DX is an MRI-conditional system that includes the MRI AutoDetect feature, which provides a dedicated MRI sensor that, when activated, provides a 14-day scanning window. During the MRI, when the dedicated sensor detects a magnetic resonance field ≤10 millitesla (mT), the device automatically suspends tachycardia therapy and changes pacing parameters to predetermined values, but biventricular pacing support can remain functional during the MRI. During this 14-day MRI window, the patient may have as many MRI scans as needed without any further pre- or post-scan reprogramming steps.
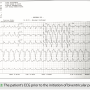
Prior to device implantation, the patient had a wide QRS of 204 ms. He was implanted with the device in June 2018. A tracing from the patient before biventricular pacing appears in Figure 2. The implantation procedure was uneventful and the patient responded well to CRT. At discharge, he had a QRS duration of 126 ms, 99% biventricular pacing, 100% CRT pacing, and a 22 mV p-wave amplitude (the generator amplifies the intrinsic p-wave signal by a factor of 4).
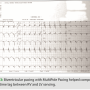 At implant, a RV to LV conduction test was performed and determined a significant 188 ms lag between RV and LV sensing. Due to the complexity of the patient and to encourage optimal response to CRT, multipole pacing was activated. BIOTRONIK offers multiple LV paces (MPP) per cardiac cycle in the form of MultiPole Pacing, allowing two pacing activation sites to be programmed independently of each other with the Sentus IS4 LV lead. The first pacing site was selected as LV4 to RV coil with a threshold of 1.2 volts at 0.75 ms. To promote the greatest distance of separation of activation, LV1 to RV coil (1.5 volts at 0.75 ms) was chosen as the second LV pacing activation site. The multipole pacing sequence was programmed with first LV (LV4 to RV coil) 25 ms prior to the second LV pacing sequence (LV1 to RV coil). LV first (MultiPole Pacing LV1 then LV2) prior to RV pacing by 30 ms was also programmed. (Figure 3)
At implant, a RV to LV conduction test was performed and determined a significant 188 ms lag between RV and LV sensing. Due to the complexity of the patient and to encourage optimal response to CRT, multipole pacing was activated. BIOTRONIK offers multiple LV paces (MPP) per cardiac cycle in the form of MultiPole Pacing, allowing two pacing activation sites to be programmed independently of each other with the Sentus IS4 LV lead. The first pacing site was selected as LV4 to RV coil with a threshold of 1.2 volts at 0.75 ms. To promote the greatest distance of separation of activation, LV1 to RV coil (1.5 volts at 0.75 ms) was chosen as the second LV pacing activation site. The multipole pacing sequence was programmed with first LV (LV4 to RV coil) 25 ms prior to the second LV pacing sequence (LV1 to RV coil). LV first (MultiPole Pacing LV1 then LV2) prior to RV pacing by 30 ms was also programmed. (Figure 3)
 The patient was discharged home and continued his optimal pharmacological therapy with equipment to allow for BIOTRONIK Home Monitoring®, which provides daily automatic remote monitoring that includes heart failure diagnostic trends. A few weeks after returning home, Home Monitoring alerted the clinic that the patient’s percentage of biventricular pacing had dropped markedly to 77% with a mean heart rate of 109 beats per minute (Figure 4). The clinical team then checked and found a drop in thoracic impedance, which suggests fluid accumulation.
The patient was discharged home and continued his optimal pharmacological therapy with equipment to allow for BIOTRONIK Home Monitoring®, which provides daily automatic remote monitoring that includes heart failure diagnostic trends. A few weeks after returning home, Home Monitoring alerted the clinic that the patient’s percentage of biventricular pacing had dropped markedly to 77% with a mean heart rate of 109 beats per minute (Figure 4). The clinical team then checked and found a drop in thoracic impedance, which suggests fluid accumulation.
 The patient was contacted and informed, and it was found he had stopped taking his diuretic. His drug therapy was re-evaluated and the patient was counseled about adherence to diuretic therapy. The patient resumed taking the diuretic, and reports showing 98% biventricular pacing, 100% CRT, and thoracic impedance trends appear in Figure 5.
The patient was contacted and informed, and it was found he had stopped taking his diuretic. His drug therapy was re-evaluated and the patient was counseled about adherence to diuretic therapy. The patient resumed taking the diuretic, and reports showing 98% biventricular pacing, 100% CRT, and thoracic impedance trends appear in Figure 5.
Discussion
CRT confers an all-cause mortality benefit for HF patients, and it has also been shown to significantly reduce the risk of HF hospitalization. The odds ratio (OR) for all-cause mortality decreases significantly and in a linear fashion with the QRS duration (P=0.009).8 In fact, patients with a wide QRS such as this patient were shown to derive particular benefits from CRT. Thus, this particular patient could derive important benefits from CRT and the decision to implant a device seems particularly appropriate.
The issue of adherence is a challenging one, not just in HF patients, but for many conditions. Patients with HF take several drugs, often multiple times a day, resulting in a high pill burden which in and of itself may contribute to nonadherence. Other conditions, such as not feeling well, being tired or groggy, or not fully understanding the drug regimen, further contribute to poor compliance. Many patients object to taking diuretics because of the bother of frequent urination. In this particular case, poor adherence led to a rapid decline in biventricular pacing which alerted the clinical team. This demonstrates the benefit of daily Home Monitoring in this particular patient population.
Finally, morbid obesity can make HF treatment more complex. As obesity is on the rise in the U.S. and other parts of the world, this problem is likely to occur more frequently in the coming years. This particular patient had a very large heart with a long QRS duration and a long delay between RV and LV sensing (188 ms). This made him an ideal candidate for CRT, but a somewhat unusual lead was required in that he needed larger electrode spacing than is typical as his heart was enlarged.
The great challenge in treating this patient is assuring his adherence to drug therapy, which remains a crucial component of successful care. It is likely that over time, CRT will prove beneficial to him in addition to providing the security of rescue defibrillation if needed. Home Monitoring and diligent clinical care can support this young man in pharmacological compliance and long-term HF management.
Conclusion
HF is a prevalent condition that can be difficult to manage, but in a subset of patients (notably those with a long QRS duration), device-based CRT can be an important part of overall care. CRT does not preclude drug therapy; indeed, the two must work together. In this particular case, the patient had a high BMI that required a specific type of defibrillation lead, but could otherwise benefit from device therapy plus optimal pharmacological therapy. Like many HF patients, the patent struggled with compliance, but Home Monitoring allowed the clinic to help monitor and advise this patient, and possibly drive better adherence.
Disclosure: The author has no conflicts of interest to report regarding the content herein.
References
- Ross JS, Chen J, Lin Z, et al. Recent national trends in readmission rates after heart failure hospitalization. Circ Heart Fail. 2010;3(1):97-103.
- Roger VL, Go AS, Lloyd-Jones DM, et al. Executive summary: heart disease and stroke statistics--2012 update: a report from the American Heart Association. Circulation. 2012;125(1):188-197.
- Ebong IA, Goff DC Jr, Rodriguez CJ, Chen H, Bertoni AG. Mechanisms of heart failure in obesity. Obes Res Clin Pract. 2014;8(6):e540-548.
- Heck PM, Dutka DP. Insulin resistance and heart failure. Curr Heart Fail Rep. 2009;6(2):89-94.
- Alpert MA, Lavie CJ, Agrawal H, Aggarwal KB, Kumar SA. Obesity and heart failure: epidemiology, pathophysiology, clinical manifestations, and management. Transl Res. 2014;164(4):345-356.
- Alpert MA, Karthikeyan K, Abdullah O, Ghadban R. Obesity and Cardiac Remodeling in Adults: Mechanisms and Clinical Implications. Prog Cardiovasc Dis. 2018 Jul 7. [Epub ahead of print]
- Davis EM, Packard KA, Jackevicius CA. The pharmacist role in predicting and improving medication adherence in heart failure patients. J Manag Care Spec Pharm. 2014;20(7):741-755.
- Kang SH, Oh IY, Kang DY, et al. Cardiac resynchronization therapy and QRS duration: systematic review, meta-analysis, and meta-regression. J Korean Med Sci. 2015;30(1):24-33.
This article is published with support from BIOTRONIK.