Left Atrial Appendage Exclusion Using a LARIAT Device: A Case Study
Introduction
Patients with atrial fibrillation (AF) face an increased risk of systemic thromboembolism.1 The left atrial appendage (LAA), a remnant of the primordial left atrium, represents the major source of cardiac thrombus formation responsible for systemic thromboembolism in patients with AF.2,3 Percutaneous transcatheter exclusion of the LAA is an important strategy to virtually eliminate the thromboembolic risk of AF and avoid the need for long-term oral anticoagulant therapy. This technique has been developed as an alternative to surgical ligation or amputation, which have been demonstrated very effective in reducing the risk of thromboembolism associated with AF.
Until recently, all available devices for percutaneous closure of the LAA, such as the Percutaneous LAA Transcatheter Occlusion (PLAATO, ev3 Inc., Plymouth, MN), the WATCHMAN® LAA closure device (Boston Scientific/Atritech, Inc., Plymouth, MN) and the AMPLATZER® Cardiac Plug (AGA Medical Corp., Plymouth, MN) device, were designed to obliterate the ostium of the LAA endocardially.
Recently, an epicardially-deployed percutaneous LAA ligation device (LARIAT®, SentreHEART, Redwood City, CA) has been released. The LARIAT® is the first percutaneous device capable to ligate — rather than occlude — the LAA, possibly replicating the benefits of surgical LAA ligation.
Case Study
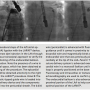 A 76-year-old woman with AF, hypertension, congestive heart failure, and chronic renal failure presented for evaluation. She had a recent history of major bleeding from the gastrointestinal tract due to peptic ulcer disease. The calculated CHA2DS2-VaSC score was 5, and the HAS-BLED score was 4.4 The patient was deemed at high risk of thromboembolism and bleeding, and unsuitable for long-term oral anticoagulant therapy.4 A percutaneous ligation of the LAA with the LARIAT® device was planned.
A 76-year-old woman with AF, hypertension, congestive heart failure, and chronic renal failure presented for evaluation. She had a recent history of major bleeding from the gastrointestinal tract due to peptic ulcer disease. The calculated CHA2DS2-VaSC score was 5, and the HAS-BLED score was 4.4 The patient was deemed at high risk of thromboembolism and bleeding, and unsuitable for long-term oral anticoagulant therapy.4 A percutaneous ligation of the LAA with the LARIAT® device was planned.
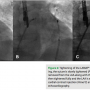 Before the procedure, a transesophageal echocardiogram was used to document the absence of thrombus in the LAA, and a cardiac CT was performed to study the anatomy of the LAA (see below). A 12 Fr LARIAT® device was successfully deployed without complication. The procedure was performed percutaneously using an epicardial approach under fluoroscopic imaging, as described by Sosa et al (see below).5 Intraoperative Doppler echocardiography and angiographic contrast injections were utilized to confirm optimal LAA ligation.
Before the procedure, a transesophageal echocardiogram was used to document the absence of thrombus in the LAA, and a cardiac CT was performed to study the anatomy of the LAA (see below). A 12 Fr LARIAT® device was successfully deployed without complication. The procedure was performed percutaneously using an epicardial approach under fluoroscopic imaging, as described by Sosa et al (see below).5 Intraoperative Doppler echocardiography and angiographic contrast injections were utilized to confirm optimal LAA ligation.
Discussion
Oral anticoagulation therapy has been consistently demonstrated as effective in preventing thromboembolic events in patients with AF. Unfortunately, some patients are not suitable for long-term oral anticoagulant therapy due to an unacceptably high risk of bleeding. Based on the empirical observation that most of the thrombi originate in the LAA,6 several surgical and percutaneous techniques have been developed with the aim of excluding the LAA from the systemic circulation. Among these, the LARIAT® LAA ligation device is the most recent one.
Technical details
 The LARIAT® system consists of three components: 1) a 20-mm compliant occlusion balloon, 2) 0.025” and 0.035” magnet-tipped guide wires, and 3) a 12 Fr suture delivery device.7,8 Indications to the LARIAT® device are similar to those of other available devices for LAA exclusion. In general, LAA ligation/exclusion is most reasonably indicated for patients with significant risk of thromboembolic complications who are unable to tolerate anticoagulants because of increased risk of bleeding complications or poor compliance. Key exclusion criteria for LARIAT® implantation include anatomical variants of the LAA and contraindications to percutaneous pericardial access (Table 1).
The LARIAT® system consists of three components: 1) a 20-mm compliant occlusion balloon, 2) 0.025” and 0.035” magnet-tipped guide wires, and 3) a 12 Fr suture delivery device.7,8 Indications to the LARIAT® device are similar to those of other available devices for LAA exclusion. In general, LAA ligation/exclusion is most reasonably indicated for patients with significant risk of thromboembolic complications who are unable to tolerate anticoagulants because of increased risk of bleeding complications or poor compliance. Key exclusion criteria for LARIAT® implantation include anatomical variants of the LAA and contraindications to percutaneous pericardial access (Table 1).
The latter should be anteriorly directed, given the anterior position of the LAA. Once the pericardial access is obtained, a 14 Fr soft-tipped epicardial guide cannula (SentreHEART, Inc.) is advanced in the pericardial space. At this stage, unfractionated heparin (adjusted-dose based on the activated clotting time [ACT]) is given. A 20-mm occlusion balloon (EndoCATH®, SentreHEART, Inc.) is loaded with the magnet-tipped 0.025” guide wire (FindrWIRZ®, SentreHEART, Inc.) and positioned in the LAA through a percutaneous transseptal approach using a 8.5 Fr SL1 transseptal sheath (St. Jude Medical, St. Paul, MN). In our institution, intracardiac echocardiography (ICE) is adopted to guide the transseptal puncture. At this stage, the 0.035” wire is then advanced in the pericardial space.
Once the two wires are in close proximity, they are magnetically guided to meet end to end; the snare is then advanced over the 0.035” epicardial wire and positioned over the LAA. The optimal positioning of the snare (i.e., ostium of the LAA) is guided by the EndoCATH® balloon location and validated through intraoperative transesophageal echocardiography. The suture device is composed of a size 0 Teflon-coated, braided polyester suture that is mounted on the delivery device in a pre-tied loop, which has a maximum diameter of 40 mm. As the suture is slowly tightened, the balloon is deflated and removed from the LAA along with the guide wire. The suture is then completely tightened to complete the LAA ligation.
Conclusions
In the patient presented in the case, the LARIAT® device was successfully deployed without any complication. Transesophageal echocardiography one month after the procedure confirmed complete LAA sealing. In conclusion, we present an illustrative case of a patient with AF not suitable for chronic oral anticoagulant therapy. She was treated successfully with a LARIAT® LAA suture device. Thus far, our experience with this device holds promise. However, future trials are necessary to better evaluate the benefits of this novel percutaneous LAA exclusion device.
References
- Wolf PA, Abbott RD, Kannel WB. Atrial fibrillation as an independent risk factor for stroke: The Framingham Study. Stroke 1991;22:983-988.
- Leung DY, Black IW, Cranney GB, et al. Prognostic implications of left atrial spontaneous echo contrast in nonvalvular atrial fibrillation. J Am Coll Cardiol 1994;24:755-762.
- Manning WJ, Silverman DI, Katz SE, et al. Impaired left atrial mechanical function after cardioversion: Relation to the duration of atrial fibrillation. J Am Coll Cardiol 1994;23:1535-1540.
- Camm AJ, Kirchhof P, Lip GY, et al. Guidelines for the management of atrial fibrillation: The Task Force for the Management of Atrial Fibrillation of the European Society of Cardiology (ESC). Europace 2010;12:1360-1420.
- Sosa E, Scanavacca M, d’Avila A, Pilleggi F. A new technique to perform epicardial mapping in the electrophysiology laboratory. J Cardiovasc Electrophysiol 1996;7:531-536.
- Blackshear JL, Odell JA. Appendage obliteration to reduce stroke in cardiac surgical patients with atrial fibrillation. Ann Thorac Surg 1996;61:755-759.
- Lee RJ, Bartus K, Yakubov SJ. Catheter-based left atrial appendage (LAA) ligation for the prevention of embolic events arising from the LAA: Initial experience in a canine model. Circ Cardiovasc Interv 2010;3:224-229.
- Singh SM, Dukkipati SR, d’Avila A, et al. Percutaneous left atrial appendage closure with an epicardial suture ligation approach: A prospective randomized pre-clinical feasibility study. Heart Rhythm 2010;7:370-376.











