Integration and Evaluation of the Cryoballoon System into a Community Hospital for the Treatment of Atrial Fibrillation
INTRODUCTION
To date, Medtronic’s Arctic Front cryoballoon system has been used in more than 70,000 procedures globally (Medtronic, Inc. database) for the catheter ablation of atrial fibrillation (AF). A variety of worldwide evaluations on the cryoballoon system have been conducted to examine safety and efficacy in different countries and healthcare systems.1-4 In summarizing these data, the cryoballoon system is typically able to achieve acute pulmonary vein isolation (PVI) in better than 90% of all procedures. Additionally, when the patient population is predominately burdened with paroxysmal AF, the one- and two-year freedom from AF can be upwards of 80% and 60%, respectively.1-3 When complications were recorded in these studies, the most common event was phrenic nerve palsy (PNP), which occurred in 2–11% of all cryoballoon procedures.2-4
In the U.S., the STOP AF clinical trial demonstrated a 69.9% cryoballoon treatment success at 12 months with 83% of the procedures achieving PVI while using only the balloon catheter.5 Similar to the worldwide experience, PNP was the prevalent adverse event that occurred in 12.3% of all cryoballoon treatments followed by new atrial flutter (AFL) and pulmonary vein (PV) stenosis, which occurred in 3.5% and 3.1% of all STOP AF cryoballoon treatments, respectively. With roughly three years of FDA-approved cryoballoon usage, we eagerly await and expect additional U.S. single-center studies to be published in the near term. Until then, a U.S. multicenter study by Johnson et al recorded and examined cryoballoon procedural efficiencies that were enhanced when physicians migrated to the second-generation cryoballoon ablation catheter (Arctic Front Advance™; Medtronic, Inc.).6 In brief, the Johnson et al study demonstrated a better than 20% reduction in procedure time, left atrial dwell time, and fluoroscopy time when the Arctic Front Advance catheter was employed. However, it has yet to be determined if these catheter improvements will translate into better long-term efficacy and/or safety. In this current examination, we evaluate our own experience with the cryoballoon system to review safety, efficacy, and efficiency during a one-year period of usage.
THE MERCY HOSPITAL EXPERIENCE
The electrophysiology (EP) department at Mercy Hospital recently set out to evaluate the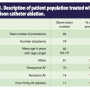 cryoballoon procedure under the “real world” conditions of a large community hospital. Mercy Hospital, in Coon Rapids, Minnesota, is a 271-bed tertiary care center and part of the Allina Health system. We started our cryoballoon program in June 2011. In this examination, we evaluated 79 consecutive patients (73% male, mean age 60 years) for whom we had one-year clinical outcomes and procedural data from their 84 procedures (Table 1). The cohort was comprised of all cryoballoon procedures conducted at Mercy Hospital during 2012. This study was performed as part of our quality surveillance efforts within the broader cardiovascular program in the Allina Health system of networked hospitals.
cryoballoon procedure under the “real world” conditions of a large community hospital. Mercy Hospital, in Coon Rapids, Minnesota, is a 271-bed tertiary care center and part of the Allina Health system. We started our cryoballoon program in June 2011. In this examination, we evaluated 79 consecutive patients (73% male, mean age 60 years) for whom we had one-year clinical outcomes and procedural data from their 84 procedures (Table 1). The cohort was comprised of all cryoballoon procedures conducted at Mercy Hospital during 2012. This study was performed as part of our quality surveillance efforts within the broader cardiovascular program in the Allina Health system of networked hospitals.
In this study, 83% of the patients had paroxysmal AF and 17% had persistent AF symptoms at the time of treatment. Of these patients, 13% had undergone a prior AF ablation using focal radiofrequency (RF) catheters. During their course of cryoballoon treatment(s), each patient received a cryoballoon PVI, and when needed, RF catheter ablation was utilized as an adjunctive tool for any remaining atrial tachycardias (ATs) or flutters.
Table 2 lists complications which included tamponade (4%), PNP (1%), and PV stenosis (1%). Most of the tamponade cases resulted from difficulties related to a second transseptal access, after the 15 Fr cryoballoon sheath (FlexCath®; Medtronic, Inc.) was already in place. By January 2013 (after the study period discussed in this publication), we had adopted a single transseptal approach for our AF cryoablation procedures. Since this switch, there has been only one tamponade in over 108 additional cryoballoon procedures (0.9% event rate). All tamponades were recognized and treated during the procedure without further sequelae.
With regard to PV stenosis and PNP, the PV stenosis was a late onset complication that was confirmed by radiological examination for dyspnea during patient follow-up cardiac care. While a PV stenosis was measurable by imaging, the patient did not require stenting or any further surgical intervention. The single instance of PNP was detected with diaphragmatic pacing during the right-sided PV cryoablation procedure, but the phrenic nerve interruption (PNI) did resolve before the next-day patient discharge. Neither patient had further complications when examined at one-year follow-up.
during the right-sided PV cryoablation procedure, but the phrenic nerve interruption (PNI) did resolve before the next-day patient discharge. Neither patient had further complications when examined at one-year follow-up.
During the 12-month follow-up care examinations of these 79 patients (66 paroxysmal AF and 13 persistent AF), eight patients with paroxysmal AF (10%) required a second AF ablation and three of these eight patients also received an ablation for AFL or AT during this second procedure (Table 3). One patient with initial paroxysmal AF did require a second ablation treatment for only AFL. In total, 57 patients with paroxysmal AF (86%) were free of AF at 12 months and only six of those responding (AF free) patients were still taking anti-arrhythmic drug (AAD) medication during the 12-month examination point. Those six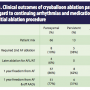 patients still on AADs were being drug treated for other arrhythmias, including premature ventricular contractions, inappropriate sinus tachycardias, and ATs. At 12-month follow-up, 51 patients (77%) with previous paroxysmal AF were free of AF and did not require any further AADs. While only 13 patients with persistent AF were examined, eight patients (62%) remained free of AF and four of those responders (31%) were off of all AADs. Additionally, no patient (starting with a persistent AF diagnosis) needed any further ablation of AFL or AT during a later ablation.
patients still on AADs were being drug treated for other arrhythmias, including premature ventricular contractions, inappropriate sinus tachycardias, and ATs. At 12-month follow-up, 51 patients (77%) with previous paroxysmal AF were free of AF and did not require any further AADs. While only 13 patients with persistent AF were examined, eight patients (62%) remained free of AF and four of those responders (31%) were off of all AADs. Additionally, no patient (starting with a persistent AF diagnosis) needed any further ablation of AFL or AT during a later ablation.
During this study period, we examined certain procedural parameters as part of our quality surveillance efforts for the hospital (Table 4). These 84 procedures were separated into four groups of 21 patients each (in chronological order) in order to examine procedural efficiencies and patterns of usage that had occurred during our one-year cryoballoon experience. With regard to better patient safety, fluoroscopy time and dose progressively showed reduction from Group 1 to Group 4. Similarly, with regard to hospital resource efficiency, both procedure time and the percentage of general anesthesia procedures had also demonstrated a progressive reduction from Group 1 to Group 4. For fluoroscopy time and percentage of general anesthesia usage, the change from Group 1 to Group 4 was statistically significant when tested by 2-tailed, 2-sample, t-test (p-value = 0.002 and p-value = 0.001, respectively). Although no formal data was displayed in Table 4, we estimate that our physician time had decreased from approximately 4 hours to 3 hours when comparing cases in Group 1 to Group 4.
groups of 21 patients each (in chronological order) in order to examine procedural efficiencies and patterns of usage that had occurred during our one-year cryoballoon experience. With regard to better patient safety, fluoroscopy time and dose progressively showed reduction from Group 1 to Group 4. Similarly, with regard to hospital resource efficiency, both procedure time and the percentage of general anesthesia procedures had also demonstrated a progressive reduction from Group 1 to Group 4. For fluoroscopy time and percentage of general anesthesia usage, the change from Group 1 to Group 4 was statistically significant when tested by 2-tailed, 2-sample, t-test (p-value = 0.002 and p-value = 0.001, respectively). Although no formal data was displayed in Table 4, we estimate that our physician time had decreased from approximately 4 hours to 3 hours when comparing cases in Group 1 to Group 4.
At the outset of our balloon cryothermy program, we knew we wanted the assistance of our anesthesia colleagues, but we also knew we wanted to wean ourselves from depending on them entirely. They had limited resources and could only offer coverage two days a week. As the year progressed in 2012, our team felt we were ready to take on these cases with conscious sedation administered by our dedicated EP nursing team (as seen in Table 4). We found this improved care by allowing for faster in-hospital recovery, reduced usage of Foley catheters (thus diminishing the risk for hospital-borne infections), and lessened post-operative discomfort related to endotracheal intubation. Furthermore, we were able to further efficiencies and patient satisfaction by offering more potential scheduling time slots (including scheduling multiple PVI procedures in a day without staff overtime) and cost savings from avoiding use of general anesthesia (and any additional billing by an anesthesia provider). Our anesthesia colleagues continue to provide coverage when patient factors deem that it is the best route. They also provide quality oversight to our EP nurses administering conscious sedation, and remain valuable advocates for our program and the safety of our patients. In general, our patients have minimal to no recall of the ablation procedure, even if there is occasional evidence for discomfort during freezing, such as a rise in blood pressure or patient movement.
DISCUSSION
This evaluation of balloon cryothermy for treatment of AF at Mercy Hospital demonstrated the efficacy and safety of the procedure when applied by an experienced and dedicated team. During this early phase of our experience, we observed a low complication rate. The incidence of PNP at 1% was much lower than the STOP AF trial and amongst the lowest from previously published data. Although PNI can still be challenging when using the cryoballoon, the biological characteristics and pathways of developing PNI are becoming better understood.7 Histological examination has demonstrated that the cryo-induced damage of the large myelinated axons by Wallerian degeneration first shows evidence of degradation followed by axonal re-myelination and regeneration. Additionally, there have been several new and creative methods to monitor phrenic nerve function during a cryoablation,8-11 and these methods typically involve assessing direct phrenic nerve function with diaphragmatic electromyography or direct diaphragm imagining for motion assessment.
Our low PNP incidence rate was accomplished by vigilant sight and tactile observations of diaphragmatic contractions when pacing the phrenic nerve on right-sided left atrial cryoablations. In addition to a technician constantly palpating the right chest wall for diaphragmatic contractions, we observe the diaphragmatic contractions via intracardiac ultrasound either from the right atrium or the hepatic vein. Intracardiac ultrasound is used on all cases to also facilitate transseptal puncture, determine PV location and ostial dimension/morphology, assess for cryoballoon-to-PV occlusion, and monitor for effusion or thrombi.
New ATs were present in our study at an incidence of 5% and were consistent with the 3.5% incidence that was found during the STOP AF trial after the initial cryoballoon procedure. Both were in agreement with an extensive cryoballoon study conducted by Mikhaylov et al, which found an 8% incidence of ATs after cryoballoon ablation with no documentation of any left atrial macro-reentrant tachycardia.12 Non-transmural lesions around PVs are a major cause of and risk factor for developing new ATs after radiofrequency catheter ablation.13 Our low incidence of post-ablation ATs are consistent with cryoballoon lesions that are transmural and durable.14 Additionally, our 1% PV stenosis rate in this study was in general agreement with the 3.1% stenosis rate found in all cryoballoon-treated patients during the STOP AF clinical trial.5 Importantly, the patients in the STOP AF study had undergone chest imaging for PV stenosis regardless of symptoms. In every case, an antral placement of the balloon catheter during ablation can potentially decrease the risk of PV stenosis.
found an 8% incidence of ATs after cryoballoon ablation with no documentation of any left atrial macro-reentrant tachycardia.12 Non-transmural lesions around PVs are a major cause of and risk factor for developing new ATs after radiofrequency catheter ablation.13 Our low incidence of post-ablation ATs are consistent with cryoballoon lesions that are transmural and durable.14 Additionally, our 1% PV stenosis rate in this study was in general agreement with the 3.1% stenosis rate found in all cryoballoon-treated patients during the STOP AF clinical trial.5 Importantly, the patients in the STOP AF study had undergone chest imaging for PV stenosis regardless of symptoms. In every case, an antral placement of the balloon catheter during ablation can potentially decrease the risk of PV stenosis.
The 86% one-year freedom from AF in our paroxysmal AF patients was better than the STOP AF results,5 but in line with other cryoballoon worldwide experiences.1,2 The small group of persistent AF patients were able to achieve a 62% one-year freedom from AF response when treated at our center. However, this rather large differential response had led our group to recognize that earlier intervention is likely to lead to better outcomes. We conduct local cardiology seminars on the importance of early AF intervention, and our general suggestions have been that we are advocates for treating patients by catheter ablation who fit the following criteria: paroxysmal AF, age less than 75 years, left atrial dimension of less than 5 cm (AP direction), and cannot tolerate or have failed at least one AAD.
CONCLUSION
The intent of this study was to examine the cryoballoon AF ablation program at Mercy Hospital for efficacy, safety, and hospital efficiency, as part of our quality surveillance efforts within the cardiovascular program. We estimate that based on efficiencies achieved in staff labor, materials, hospital resources, and elimination of general anesthesia, our AF cryoablation procedures in 2012 cost approximately 20–30% less compared to PVIs in 2011, when we were predominantly utilizing RF ablations. Recently, we have embarked upon a more systematic approach to analyzing these cost savings and hope to make these data available in publication form once the final analyses are complete.
Certainly, our adoption of the cryoballoon system is not the sole cause for these efficiencies. In fact, contrary to other centers that employ popular cryoballoon usage without 3D mapping, we utilize 3D electroanatomical mapping for all our PVI cases. We find that it cuts down on fluoroscopy usage, enables ablation of ATs and complex fractionated atrial electrograms (when present), and has given us new insight into the amount of atrial scarring in AF ablation procedures.
With the cryoballoon system in paroxysmal patients, we rarely need an adjunctive focal RF ablation catheter to supplement PVI. Additionally, the use of conscious sedation rather than general anesthesia has decreased cost and operational complexity of the procedure. Lastly, the straightforward predictability of the cryoballoon procedures has allowed the EP group to schedule two AF ablation cases per day with a high degree of certainty that both cases will finish without requiring staff overtime. As we begin to analyze our 2013 experience with the cryoballoon system, we rarely encounter a case that is longer than 4 hours in total procedure time. Most straightforward cases are done in 2.5 to 3 hours, with less than ten minutes of fluoroscopy. These changes have helped us maintain patient safety and efficacy while still practicing AF ablation in a cost conscious and effective manner.
Disclosures: Dr. Nguyen has no conflicts of interest to report. Hae Lim, PhD declares a competing financial interest; he is an employee of Medtronic, Inc., a publicly traded company. Outside the submitted work, Dr. Lin reports consultancy with Medtronic regarding education of physicians regarding cryoablations, and reports travel/accomodations expenses covered or reimbursed by Medtronic for cryoablation conference; Dr. Remole reports consultancy with Medtronic as a group instructor and proctor, and reports travel/accomodations expenses covered or reimbursed by Medtronic for course instruction.
References
- Aytemir K, Oto A, Canpolat U, et al. Immediate and medium-term outcomes of cryoballoon-based pulmonary vein isolation in patients with paroxysmal and persistent atrial fibrillation: single-centre experience. J Interv Card Electrophysiol. 2013;38(3):187-195.
- Ferrero-de Loma-Osorio A, Izquierdo-de Francisco M, Martínez-Brotons A, et al. Medium-term results of cryoballoon ablation of the pulmonary veins in patients with paroxysmal and persistent atrial fibrillation. First experience of a Spanish center. J Interv Card Electrophysiol. 2013;37(2):189-196.
- Vogt J, Heintze J, Gutleben KJ, et al. Long-term outcomes after cryoballoon pulmonary vein isolation: results from a prospective study in 605 patients. J Am Coll Cardiol. 2013;61(16):1707-1712.
- Neumann T, Wójcik M, Berkowitsch A, et al. Cryoballoon ablation of paroxysmal atrial fibrillation: 5-year outcome after single procedure and predictors of success. Europace. 2013;15(8):1143-1149.
- Packer DL, Kowal RC, Wheelan KR, et al. STOP AF Cryoablation Investigators. Cryoballoon ablation of pulmonary veins for paroxysmal atrial fibrillation: first results of the North American Arctic Front (STOP AF) pivotal trial. J Am Coll Cardiol. 2013;61(16):1713-1723.
- Johnson E, Seide H, Hackett FK, et al. A U.S. multicenter examination of the new cryoballoon: Early experience with procedural enhancements for the treatment of atrial fibrillation. EP Lab Digest. 2013;13(5):34-39.
- Andrade JG, Dubuc M, Ferreira J, et al. Histopathology of cryoballoon ablation-induced phrenic nerve injury. J Cardiovasc Electrophysiol. 2013 Sep 19. [Epub ahead of print]
- Lakhani M, Saiful F, Parikh V, et al. Recordings of diaphragmatic electromyograms during cryoballoon ablation for atrial fibrillation accurately predict phrenic nerve injury. Heart Rhythm. 2013 Nov 16. [Epub ahead of print]
- Franceschi F, Koutbi L, Mancini J, et al. Novel electromyographic monitoring technique for prevention of right phrenic nerve palsy during cryoballoon ablation. Circ Arrhythm Electrophysiol. 2013;6(6):1109-1114. [Epub 2013 Oct 10.]
- Lakhani M, Saiful F, Bekheit S, Kowalski M. Use of intracardiac echocardiography for early detection of phrenic nerve injury during cryoballoon pulmonary vein isolation. J Cardiovasc Electrophysiol. 2012;23(8):874-876.
- Franceschi F, Dubuc M, Guerra PG, Khairy P. Phrenic nerve monitoring with diaphragmatic electromyography during cryoballoon ablation for atrial fibrillation: the first human application. Heart Rhythm. 2011;8(7):1068-1071.
- Mikhaylov EN, Bhagwandien R, Janse PA, et al. Regular atrial tachycardias developing after cryoballoon pulmonary vein isolation: incidence, characteristics, and predictors. Europace. 2013;15(12):1710-1717.
- Calkins H, Kuck KH, Cappato R, et al. 2012 HRS/EHRA/ECAS expert consensus statement on catheter and surgical ablation of atrial fibrillation. Europace. 2012;14(4):528-606.
- Ahmed H, Neuzil P, Skoda J, et al. The permanency of pulmonary vein isolation using a balloon cryoablation catheter. J Cardiovasc Electrophysiol. 2010;21(7):731-737.











