An Initiative Technique to Facilitate Axillary Vein Puncture During CRT Implantation
One of the challenges to the wide application of cardiac resynchronization therapy (CRT) has been the technical difficulty encountered when obtaining venous access while utilizing an axillary venous puncture.1 This is mainly due to the vague anatomical landmarks. However, the axillary vein is the preferred access site because associated risks such as pneumothorax, mediastinal hematoma, hemothorax, and tracheal injury are relatively lower, and it facilitates better lead protection in the future.2-4 Real-time imaging of the axillary vein, including contrast media administration, may be required to prevent the associated complications.5 Implantation of the left ventricular (LV) lead during CRT usually involves contrast media administration as well in order to define the coronary venous anatomy. However, contrast nephropathy is a well-described complication of the intravascular contrast media used in a CRT procedure, and given that there is a high prevalence of renal dysfunction, diabetes, and low blood pressure in CRT candidates, the incidence of contrast nephropathy following CRT is likely to be high.6,7 Coronary venous anatomy is initially assessed routinely at our center by cannulating the coronary sinus (CS) prior to an implantation procedure using a specialized, long, pre-shaped sheath (SL3 sheath; St. Jude Medical) introduced via the femoral vein. Therefore, we attempted to design a new technique to facilitate axillary vein puncture, which in turn may minimize the contrast media requirement and the time to axillary vein puncture during implantation of CRT devices.
Methods
Patients indicated for CRT device implantation and who were voluntarily willing to give an informed consent were included in the study. A thorough physical assessment was performed. The patients received optimal explanations on CRT regarding its risks, 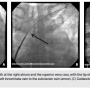 benefits, procedure length, anesthesia requirement, lead and device placement, postoperative observation, pain management, and follow-up care. The procedures were conducted in a well-equipped and adequately sterilized operating suite. Adequate conscious sedation facilitated patient comfort. As a preparation of target vein selection, we routinely perform occlusive venography to the coronary sinus (CS) using an SL3 sheath and attain the balloon via the right femoral vein. A 0.035 mm guidewire is then introduced retrogradely from the femoral vein up to the left axillary vein, passing through the inferior vena cava, right atrium, superior vena cava, left innominate vein, and left subclavian vein, supported by the SL3 sheath and its dilator. While the guidewire is in the vein, an anteroposterior (AP) scene at 7.5 frames/second is captured. The scene is then retained as a roadmap for axillary vein puncture using AP view during the implantation procedure. An SL3 sheath as well as the guidewire are then pulled back into the right atrium, in which the wire and dilator are exchanged with the attain balloon to cannulate the CS in order to perform the occlusive venography. Axillary vein punctures were attempted after device pocket preparation. Time to successful puncture was recorded. The procedure is then completed as per the protocol. Blood pressure, pulse, temperature, and pulse oximetry are monitored throughout the procedure.
benefits, procedure length, anesthesia requirement, lead and device placement, postoperative observation, pain management, and follow-up care. The procedures were conducted in a well-equipped and adequately sterilized operating suite. Adequate conscious sedation facilitated patient comfort. As a preparation of target vein selection, we routinely perform occlusive venography to the coronary sinus (CS) using an SL3 sheath and attain the balloon via the right femoral vein. A 0.035 mm guidewire is then introduced retrogradely from the femoral vein up to the left axillary vein, passing through the inferior vena cava, right atrium, superior vena cava, left innominate vein, and left subclavian vein, supported by the SL3 sheath and its dilator. While the guidewire is in the vein, an anteroposterior (AP) scene at 7.5 frames/second is captured. The scene is then retained as a roadmap for axillary vein puncture using AP view during the implantation procedure. An SL3 sheath as well as the guidewire are then pulled back into the right atrium, in which the wire and dilator are exchanged with the attain balloon to cannulate the CS in order to perform the occlusive venography. Axillary vein punctures were attempted after device pocket preparation. Time to successful puncture was recorded. The procedure is then completed as per the protocol. Blood pressure, pulse, temperature, and pulse oximetry are monitored throughout the procedure.
Results
Eighty-eight consecutive consenting patients (60 males and 28 females) were enrolled in the study between March 2011 and November 2013. The mean age of the patients was 57.54 ± 12.25 years. Clinical characteristics of the patients are detailed in Table 1. Of the total patients, 77% of the patients (n = 68) were diagnosed with non-ischemic dilated cardiomyopathy, a relatively small number (just 18%) were diagnosed with coronary artery disease, 14% had atrial fibrillation and 5% had congenital heart disease. The prevalence of diabetes and hypertension was similar, at 45%. Only 4 patients (4.6%) had undergone prior coronary artery bypass graft surgery, while 4 patients (4.6%) had prior percutaneous coronary intervention. Creatinine levels was 101.5 ±  48.48 µmol/L, with a mean left ventricular ejection fraction of 20.5 ± 5.96%. The mean international normalized ratio was 1.31 ± 0.53. Most of the patients (92%) were on angiotensin-converting enzyme inhibitors/angiotensin-receptor blockers and 95% were on beta-blockers. Digoxin therapy was given to 15 patients (17%).
48.48 µmol/L, with a mean left ventricular ejection fraction of 20.5 ± 5.96%. The mean international normalized ratio was 1.31 ± 0.53. Most of the patients (92%) were on angiotensin-converting enzyme inhibitors/angiotensin-receptor blockers and 95% were on beta-blockers. Digoxin therapy was given to 15 patients (17%).
Discussion
The transvenous approach has become the usual technique for CRT, with the axillary vein becoming the most desirable venous access site for that purpose because it is not associated with problems that accompany subclavian vein access, which include pneumothorax and subclavian crush syndrome.8,9 The axillary vein can be accessed by different techniques ranging from blind puncture and ultrasound utilization to the use of radiographic contrast.1,10-12 In our series, we did not use ultrasound or contrast media to aid with axillary vein access. On the other hand, placement of an LV lead in a coronary vein requires a thorough knowledge of the venous structure of the individual patient and the most reliable method of obtaining this information is by retrograde venography immediately before coronary vein lead placement. While only small quantities of contrast are required to perform coronary sinus venogram, larger contrast volumes may be used when difficult coronary sinus anatomy is encountered. Administration of radiographic contrast agents may result in an acute reduction in renal function.13-17 Cowburn et al showed that contrast volume was generally higher in patients who developed contrast nephropathy;18 hence, methods to reduce contrast-induced nephropathy should focus on both reducing contrast load and optimizing patient preparation for CRT. We routinely use a pre-shaped long sheath via the right femoral vein to cannulate the CS for occlusive venography as preparation for CRT implantation, as previously described;19 therefore, we were motivated to investigate a suitable novel technique for facilitating retrograde wiring of the axillary veins as well as subsequently reducing the time for axillary vein puncture, and probably reducing the need for contrast to delineate the axillary vein anatomy. The number of attempts to puncture the axillary vein were not counted in either group. The only limiting factor in this technique is the presence of venous valves, which require gentle manipulation to prevent vascular injury, yet we did not encounter any difficulties cannulating the axillary vein. All cases in the current series were de novo, and no previously implanted pacing leads existed in any of our cases. There were no reported vascular vein access-related complications in our series during insertion or after removal of the sheath. Performing this technique was not associated with any extra cost because the same pre-shaped sheath and guidewire were used for retrograde wiring of the axillary vein prior to CS cannulation.
The above technique was implemented successfully in 88 patients without any need for the administration of contrast media, with an axillary vein puncture time of less than 1 minute in 41% of the cases. The highlight of the technique was an absence of vascular complications and mortality in any of the patients. This technique might be useful in a subset of patients with renal impairment.
Conclusion
The use of retrograde axillary vein wiring simplifies axillary venous puncture while maintaining ample safety measures during CRT device implantation without compromising visualization of the anatomy. However, a larger prospective study is required to prove the technique’s efficacy, describe its anticipated complications, and compare it with the conventional techniques.
Disclosure: The authors report no conflicts of interest regarding the content herein.
Reprinted with permission from the Journal of Invasive Cardiology. 2015;27(7)341-343.
References
- Ramza BM, Rosenthal L, Hui R, et al. Safety and effectiveness of placement of pacemaker and defibrillator leads in the axillary vein guided by contrast venography. Am J Cardiol. 1997;80:892-896.
- Smith BE, Modell JH, Gaub ML, et al. Complications of subclavian vein catheterization. Arch Surg. 1965;90:228-229.
- Morgan RNW, Morrell DF. Internal jugular catheterisation: a review of a potentially lethal hazard. Anaesthesia. 1981;36:512-517.
- Burri H, Sunthorn H, Dorsaz PA, et al. Prospective study of axillary vein puncture with or without contrast venography for pacemaker and defibrillator lead implantation. Pacing Clin Electrophysiol. 2005;28:S280-S283.
- Sandhu NS. Transpectoral ultrasound-guided catheterization of the axillary vein: an alternative to standard catheterization of the subclavian vein. Anesth Analg. 2004;99:183-187.
- Ramza BM, Rosenthal L, Hui R, et al. Safety and effectiveness of placement of pacemaker and defibrillator leads in the axillary vein guided by contrast venography. Am J Cardiol. 1997;80:892-896.
- Kohli A. Contrast-induced nephropathy (CIN): can we minimize its effects? Ind J Radiol Imag. 2005;15:307-308.
- Fyke FE III. Infraclavicular lead failure: tarnish on a golden route. Pacing Clin Electrophysiol. 1993;16:373-376.
- Magney JE, Flynn DM, Parsons JA, et al. Anatomical mechanisms explaining damage to pacemaker leads, defibrillator leads, and failure of central venous catheters adjacent to the sternoclavicular joint. Pacing Clin Electrophysiol. 1993;16:445-447.
- Byrd CL. Safe introducer technique for pacemaker lead implantation. Pacing Clin Electrophysiol. 1992;15:262-267.
- Byrd CL. Clinical experience with the extrathoracic introducer insertion technique. Pacing Clin Electrophysiol. 1983;16:1781-1784.
- Spencer WK III, Zhu DWX, Kirkpatrick C, Killip D, Durand JB. Subclavian venogram as a guide to lead implantation. Pacing Clin Electrophysiol. 1998;21:499-502.
- Parfrey PS, Griffiths SM, Barrett BJ, et al. Contrast material-induced renal failure in patients with diabetes mellitus, renal insufficiency, or both: a prospective controlled study. N Engl J Med. 1989;320:143-149.
- Rich MW, Crecelius CA. Incidence, risk factors, and clinical course of acute renal insufficiency after cardiac catheterization in patients 70 years of age or older: a prospective study. Arch Intern Med. 1990;150:1237-1242.
- Schwab SJ, Hlatky MA, Pieper KS, et al. Contrast nephrotoxicity: a randomized controlled trial of a non-ionic and an ionic radiographic contrast agent. N Engl J Med. 1989;320:149-153.
- Stevens MA, McCullough PA, Tobin KJ, et al. A prospective randomized trial of prevention measures in patients at high risk for contrast nephropathy: results of the PRINCE study: prevention of radiocontrast induced nephropathy clinical evaluation. J Am Coll Cardiol. 1999;33:403-411.
- Solomon R, Werner C, Mann D, et al. Effects of saline, mannitol, and furosemide on acute decreases in renal function induced by radiocontrast agents. N Engl J Med. 1994;331:1416-1420.
- Cowburn PJ, Patel H, Pipes RR, et al. Contrast nephropathy post cardiac resynchronization therapy: an under-recognized complication with important morbidity. Eur J Heart Failure. 2005;7:899-903.
- Al-Khadra AS. Use of pre-shaped sheath to plan and facilitate cannulation of the coronary sinus for the implantation of cardiac resynchronization therapy devices. Pacing Clin Electrophysiol. 2005;28:489-492.











