Fluoroscopy-Free Ablation of SVT Using 3D Mapping
Background
Fluoroscopy has historically been the primary imaging modality used in percutaneous interventional procedures. The use of ionizing radiation puts patients and staff at risk of the deterministic and stochastic effects of radiation exposure.
Alternative imaging systems, including 3D electroanatomic mapping systems and ultrasound, can be used alone or in conjunction with traditional fluoroscopy to reduce radiation exposure. Utilizing the St. Jude Medical EnSite Velocity NavX 3D mapping system, we have performed successful ablations of supraventricular tachycardia (SVT) and idiopathic ventricular tachycardia (VT) without use of fluoroscopy. This method has allowed patients, physicians and staff to undergo the procedure without radiation exposure, and frees the physician and staff from wearing lead aprons.
The case presented here is a description of our typical SVT ablation for AV nodal reentrant tachycardia (AVNRT) performed using EnSite mapping without fluoroscopy at the University of Wisconsin Hospital.
Case Description
A 26-year-old female presented to our institution with a history of palpitations associated with chest discomfort and shortness of breath for more than 13 years. Holter and event monitoring demonstrated episodes of a short-RP narrow complex tachycardia at rates greater than 200 beats per minute, occasionally with right bundle branch aberrancy. A structurally normal heart was confirmed by echocardiogram. Treatment with beta-blockade was poorly tolerated, and she was referred for EP study and catheter ablation.
Placing the Diagnostic Catheters without Fluoroscopy
During the EP study, using the modified Seldinger technique, 12F Trio, 8F and 7F sheaths were placed into the right and left femoral veins. A Boston Scientific Polaris decapolar catheter was advanced through the right femoral vein to the inferior vena cava (IVC) / right atrium (RA) junction under the guidance of the EnSite Velocity NavX mapping system supplemented by 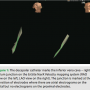 tactile feel (Figure 1). Catheters were redirected if they went into a side branch, based on medial or lateral movement on the EnSite mapping location as well as resistance when advancing the catheter. The decapolar catheter was used for initial geometry collection by the EnSite Velocity NavX mapping system in the right atrium. Using intracardiac electrograms, the superior vena cava (SVC), IVC, and tricuspid annulus were demarcated (Figure 2). The decapolar catheter was then positioned into the coronary sinus (CS) (Figure 3), confirmed by
tactile feel (Figure 1). Catheters were redirected if they went into a side branch, based on medial or lateral movement on the EnSite mapping location as well as resistance when advancing the catheter. The decapolar catheter was used for initial geometry collection by the EnSite Velocity NavX mapping system in the right atrium. Using intracardiac electrograms, the superior vena cava (SVC), IVC, and tricuspid annulus were demarcated (Figure 2). The decapolar catheter was then positioned into the coronary sinus (CS) (Figure 3), confirmed by 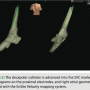 LAO and RAO orthogonal views on the 3D mapping system. The His, RA, and RV catheters (St. Jude Medical Livewire™ deflectable octapolar and fixed Josephson quadrapolars) were advanced into position using tactile feel and the EnSite mapping system to guide advancement through the venous system. Once these catheters had reached the level of the previously created RA anatomical shell, they were placed into traditional sites of high RA, RV apex, and tricuspid annulus at the level of the His (Figure 4). Orthogonal LAO and RAO views
LAO and RAO orthogonal views on the 3D mapping system. The His, RA, and RV catheters (St. Jude Medical Livewire™ deflectable octapolar and fixed Josephson quadrapolars) were advanced into position using tactile feel and the EnSite mapping system to guide advancement through the venous system. Once these catheters had reached the level of the previously created RA anatomical shell, they were placed into traditional sites of high RA, RV apex, and tricuspid annulus at the level of the His (Figure 4). Orthogonal LAO and RAO views 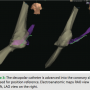 are utilized on EnSite. As the His catheter was placed, the presence of atrial or ventricular electrograms allowed the tricuspid valve to be demarcated.
are utilized on EnSite. As the His catheter was placed, the presence of atrial or ventricular electrograms allowed the tricuspid valve to be demarcated.
Pacing thresholds were checked and programmed electrical stimulation was performed. With ventricular burst pacing, the patient developed a narrow complex tachycardia with 1:1 VA conduction at a cycle length of 418 ms. Isoproterenol infusion was required to ensure stable, persistent tachycardia. The septal VA time 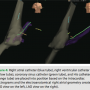 was <70 ms. The earliest atrial activation was in the proximal His catheter. RV pacing during tachycardia at a cycle length 10 ms less than the tachycardia CL entrained the tachycardia, with a V-A-V response following cessation of pacing. The diagnosis of typical AVNRT was made, and the plan was to proceed to ablation with cryo.
was <70 ms. The earliest atrial activation was in the proximal His catheter. RV pacing during tachycardia at a cycle length 10 ms less than the tachycardia CL entrained the tachycardia, with a V-A-V response following cessation of pacing. The diagnosis of typical AVNRT was made, and the plan was to proceed to ablation with cryo.
Ablation Technique
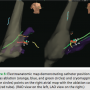 For stability of manipulation of the cryo catheter, a St. Jude Medical SRO sheath was used. To place this without fluoroscopy, first a 0.035” 50 cm wire was advanced through the short 8F sheath to a measured distance approximately to the level of IVC/RA junction. The sheath exchanged over the wire and placed into approximately the IVC just past the IVC/iliac venous junction. The dilator and wire were removed, and a 4 mm Medtronic Freezor Cardiac Cryoablation Catheter was advanced to the end of the SRO sheath. The sheath was withdrawn over the catheter until the catheter electrodes exited the sheath and were subsequently visualized on EnSite. Once the catheter was out of the sheath (when the catheter is still within the sheath, the electrode pairs are inverted and obviously deformed, making judgment of where the electrode pairs are in relation to the sheath straightforward), the two were advanced as a unit, slowly through the venous system up to the right atrium. The sheath and cryoablation catheter were then advanced to the triangle of Koch near the anatomical region of the slow pathway using the previously rendered right atrial map.
For stability of manipulation of the cryo catheter, a St. Jude Medical SRO sheath was used. To place this without fluoroscopy, first a 0.035” 50 cm wire was advanced through the short 8F sheath to a measured distance approximately to the level of IVC/RA junction. The sheath exchanged over the wire and placed into approximately the IVC just past the IVC/iliac venous junction. The dilator and wire were removed, and a 4 mm Medtronic Freezor Cardiac Cryoablation Catheter was advanced to the end of the SRO sheath. The sheath was withdrawn over the catheter until the catheter electrodes exited the sheath and were subsequently visualized on EnSite. Once the catheter was out of the sheath (when the catheter is still within the sheath, the electrode pairs are inverted and obviously deformed, making judgment of where the electrode pairs are in relation to the sheath straightforward), the two were advanced as a unit, slowly through the venous system up to the right atrium. The sheath and cryoablation catheter were then advanced to the triangle of Koch near the anatomical region of the slow pathway using the previously rendered right atrial map.
The clinical tachycardia was then re-induced. Cryomapping at -30oC resulted in tachycardia termination in the slow pathway. The PR and AH segments were monitored for stability for 45 seconds. With no evidence of PR or AH prolongation, a full ablation lesion of 240 seconds at less than -70oC was delivered. A second lesion was placed at the same site immediately after the initial ablation lesion. A significant advantage of the cryoablation technique is that upon freezing the ablation catheter adheres to the myocardium, preventing unexpected catheter tip movement and increasing the safety.1
Atrial extrastimulus testing following the second freeze demonstrated an AH discontinuity with a >50 ms increase following 10 ms decrement of atrial extrastimulus pacing. No AV nodal echo beats or tachycardia could be induced with or without isoproterenol. Further cryomapping efforts continued with the purpose of eliminating the AH discontinuity. Additional ablation lesions successfully terminated the AH jump. After 30 minutes post ablation, repeat testing and programmed stimulation were performed without evidence of an AH jump, AV nodal echoes, or tachycardia induction (Figure 5). The catheters and sheaths were withdrawn and hemostasis achieved with manual pressure. There were no complications either immediate or in follow-up. The total time for catheter placement was 5 minutes and the total procedure time was 2 hours and 12 minutes.
Discussion
This case study demonstrates our approach to fluoroscopy-free SVT ablation utilizing a 3D electroanatomic mapping system and cryoablation catheter. There are multiple providers that utilize this technique at the University of Wisconsin, and we have found that the approach has a favorable learning curve. Our institution has a greater than two-year experience, and more than 70 procedures have been performed safely without fluoroscopy.
Reduction in the complications and risks of radiation exposure to patients and medical staff is an ongoing initiative in the medical world, specifically in percutaneous interventional procedures. There currently is no data on a safe level of radiation exposure, and the current practice is to minimize and use the as low as reasonably achievable (ALARA) principle.2 Due in part to increased awareness and media attention, patients are increasingly concerned about radiation exposure during medical procedures. This is especially the case for reproductive-age women, who are a subset of the population that frequently have supraventricular arrhythmias and undergo EP procedures. Radiation exposure is not only a risk to the patient but also to the operators and staff who have additive exposure with each procedure. Fluoroscopy places additional physical stress on the laboratory staff and physicians who must wear heavy protective lead garments, which can lead to back and spine problems. Through innovative systems and new techniques, fluoroscopic times and radiation exposure for EP procedures have been decreasing.
One of the more notable advances is the use of 3D mapping systems as an adjunct to fluoroscopy. Papagiannis et al demonstrated a reduction in fluoroscopic time for SVT procedures in children, from an average of 24.9 minutes to 10.4 minutes, utilizing the EnSite NavX 3D anatomical mapping system.3 Utilizing similar techniques as our case description, Casella et al showed an even further reduction in fluoroscopic time in adults, to an average of two minutes and even zero fluoroscopy cases utilizing the EnSite system.4 Razminia and colleagues describe their experience utilizing electroanatomical mapping systems in conjunction with intracardiac echocardiography to perform ablations safely and effectively without the use of fluoroscopy.5 Other case reports and series have described SVT ablations without the use of fluoroscopy using 3D anatomical mapping systems in children and adults, demonstrating the safety and efficacy of the procedure.6-10
Systems such as the Hansen Medical robotic catheter system and Stereotaxis utilize remote navigation of catheters to decrease radiation exposure and have been utilized in SVT ablations.11 Other novel systems such as the MediGuide™ Technology (St. Jude Medical) utilize a 3D anatomical mapping system and merge traditional fluoroscopy imaging in efforts to reduce radiation exposure.12 These systems are not widely available and are an additional expense. An advantage with the electroanatomic mapping systems is that they have wider spread availability.
Performing an SVT ablation without fluoroscopy allows the operator and lab staff to not wear lead-protective garments, reducing orthopedic injuries. In a survey of physicians who perform procedures in an interventional laboratory, 42% had spine problems as compared to 27% of the general population.13,14 The effect seemed to be linear, as there was an increased incidence of spine problems related to the more procedures one did in the fluoroscopy suite.13 This suggests that decreasing the number of cases without wearing lead-protective garments would help reduce orthopedic work-related injuries.
Lastly, the EP laboratory can also be set up and designed in a more ergonomic fashion if fluoroscopy equipment is not required. Bed height, monitors, lines, and displays can be positioned in additional configurations. The fluoroless SVT ablation procedures are currently performed in our electrophysiology suite on the fluoroscopy table. However, as these techniques continue to be improved upon, it raises the possibility of performing the procedure in a number of different operating suites and beds, which would allow greater flexibility for the operators and greater comfort for the patient.
Disclosures: Dr. Oujiri, Dr. Kipp, Dr. Leal, Dr. Field, and Dr. Eckhardt have no conflicts of interest to report. Anton Bares, BS reports former employment as an FCE and stock/stock options with St. Jude Medical.
References
- Eckhardt L, Leal M, Hollis Z, Tanega J, Alberte C. Cryoablation for AVNRT: importance of ablation endpoint criteria. J Cardiovasc Electrophysiol. 2012:23;729-734.
- Committee to Assess Health Risks from Exposure to Low Levels of Ionizing Radiation. Health Risks from Exposure to Low Levels of Ionizing Radiation, BEIR VII Phase 2. 2006; The National Academies Press.
- Papagiannis J, Tsoutsinos A, Kirvassilis G, et al. Nonfluoroscopic catheter navigation for radiofrequency catheter ablation of supraventricular tachycardia in children. Pacing Clin Electrophysiol. 2006;29:971-978.
- Casella M, Pelargonio G, Dello Russo A, et al. “Near-zero” fluoroscopic exposure in supraventricular arrhythmia ablation using the EnSite NavX™ mapping system: personal experience and review of the literature. J Interv Card Electrophysiol. 2011;31:109-118.
- Razminia M, Manankil MF, Eryazici PL, et al. Nonfluoroscopic catheter ablation of cardiac arrhythmias in adults: feasibility, safety, and efficacy. J Cardiovasc Electrophysiol. 2012;23:1078-1086.
- Alvarez M, Tercedor L, Almansa I, et al. Safety and feasibility of catheter ablation for atrioventricular nodal re-entrant tachycardia without fluoroscopic guidance. Heart Rhythm. 2009;6:1714-1720.
- Gist K, Tigges C, Smith G, Clark J. Learning curve for zero-fluoroscopy catheter ablation of AVNRT: early versus late experience. Pacing Clin Electrophysiol. 2011;34:264-268.
- Tuzcu V. A nonfluoroscopic approach for electrophysiology and catheter ablation procedures using a three-dimensional navigation system. Pacing Clin Electrophysiol. 2007;30:519-525.
- Hindricks G, Willems S, Kautzner J, et al. Effect of electroanatomically guided versus conventional catheter ablation of typical atrial flutter on the fluoroscopy time and resource use: a prospective randomized multicenter study. J Cardiovasc Electrophysiol. 2009;20:734-740.
- Earley MJ, Showkathali R, Alzetani M, et al. Radiofrequency ablation of arrhythmias guided by non-fluoroscopic catheter location: a prospective randomized trial. Eur Heart J. 2006;27:1223-1229.
- Xu D, Yang B, Shan Q, et al. Initial clinical experience of remote magnetic navigation system for catheter mapping and ablation of supraventricular tachycardias. J Interv Card Electrophysiol. 2009;25:171-174.
- Sommer P, Wojdyla-Hordynska A, Rolf S, et al. Initial experience in ablation of typical atrial flutter using a novel three-dimensional catheter tracking system. Europace. 2013;15:578-581.
- Goldstein J, et al. Occupational hazards of interventional cardiologists: prevalence of orthopedic health problems in contemporary practice. Catheter Cardiovasc Interv. 2004;63:407-411.
- Klein LW, Miller DL, Balter S, et al. Occupational health hazards in the interventional laboratory: time for a safer environment. J Vasc Interv Radiol. 2009;20(7 Suppl):S278-83.











