First North American Experience Using EnSite Precision™ in Cardiac Mapping and Ablation
The introduction of electroanatomic guidance technology resulted in a paradigm shift in delivery of therapy for arrhythmia care. Advances in computer processing and electrical engineering have led to ever more complex and useful tools for the EP lab. The EnSite Precision™ Cardiac Mapping System is the latest catheter navigation and mapping system developed by Abbott. It was introduced in Europe early last year after receiving CE Mark in January 2016. It was subsequently released in Canada after Health Canada approval in October 2016.
As the first center in North America to use this new technology, we are excited to share our experience thus far.
About the Technology
The EnSite Precision system is based on its predecessor, EnSite Velocity™. Although it shares the same user interface and impedance tracking technology, our experience at Kingston General Hospital has found EnSite Precision to be a far more powerful tool in cardiac mapping and ablation. We find it is faster, more accurate with higher density maps, and performs consistently across cases.
This is achieved with a dual technology system that combines magnetic cardiac geometry creation with the impedance-based platform. Abbott developed sensor-enabled catheters that complement this system to allow real-time dual mapping. Employing specific features such as AutoMap and TurboMap, it produces a high-definition 3D map that has 27 times higher resolution than its predecessor, by collecting and assimilating tens of thousands of geometry points.
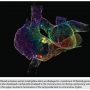 The AutoMap mapping enhancement feature records multiple simultaneous points for anatomy, voltage and activation sequence mapping. These points are taken continuously from multiple electrodes. Points that do not meet a threshold set by a list of parameters are excluded. These parameters are adjustable, including contact force minimum and maximum values, signal-to-noise ratio filter, and artifact due to respiratory movement and stability of the mapping catheter. Patient-specific filters are set according to clinical tachycardia, such as the cycle length timing and signal morphology consistency. As a result, clinically irrelevant points are automatically rejected without the need for manual removal. A common cause of rejected points would be catheter-induced ectopy. Figure 1 shows an AutoMap of an atrial tachycardia originating from the coronary sinus os.
The AutoMap mapping enhancement feature records multiple simultaneous points for anatomy, voltage and activation sequence mapping. These points are taken continuously from multiple electrodes. Points that do not meet a threshold set by a list of parameters are excluded. These parameters are adjustable, including contact force minimum and maximum values, signal-to-noise ratio filter, and artifact due to respiratory movement and stability of the mapping catheter. Patient-specific filters are set according to clinical tachycardia, such as the cycle length timing and signal morphology consistency. As a result, clinically irrelevant points are automatically rejected without the need for manual removal. A common cause of rejected points would be catheter-induced ectopy. Figure 1 shows an AutoMap of an atrial tachycardia originating from the coronary sinus os.
In addition, the TurboMap program can replay any tachycardia that occurs during the initial mapping at 10 times the normal speed. This functionality is very useful, as it can build activation maps of a PVC or tachycardia without needing to manually remap the activation sequence.
 The EnSite Precision Cardiac Mapping System has several display features that allow operators to visualize tachycardia properties and ablation results. During mapping, SparkleMap replays the tachycardia with a video representation of the activation overlaying the voltage map on the 3D geometry model (Figure 2). In cases of ventricular tachycardia and complex atrial flutter, we have found visualizing the tachycardia circuit very helpful in guiding ablation. Another feature useful in identifying ablation targets is color-coded fractionation maps, which incorporate automatically annotated fractionated electrograms.
The EnSite Precision Cardiac Mapping System has several display features that allow operators to visualize tachycardia properties and ablation results. During mapping, SparkleMap replays the tachycardia with a video representation of the activation overlaying the voltage map on the 3D geometry model (Figure 2). In cases of ventricular tachycardia and complex atrial flutter, we have found visualizing the tachycardia circuit very helpful in guiding ablation. Another feature useful in identifying ablation targets is color-coded fractionation maps, which incorporate automatically annotated fractionated electrograms.
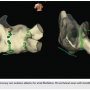 Another important feature is AutoMark, which offers color-coded designation of the lesion formation. This program collects real-time sums of contact force recordings. These calculations are based on contact force parameters studied in atrial fibrillation (AF) ablations.1-3 The AutoMark lesions represent force-time integral (FTI) or lesion index (LSI) values that can be set by the operator’s preference. The amount of ablation is represented by the size and color of the circle or sphere on the 3D model. Lesion criteria, such as contact force and spatial stability, can also be set by the user. In addition to the voltage signal, it provides a useful tool to visualize areas that might require further ablation. A pertinent example would be finding a gap in a reconnected pulmonary vein that was previously isolated. An example of AutoMark in pulmonary vein isolation (PVI) is shown in Figure 3.
Another important feature is AutoMark, which offers color-coded designation of the lesion formation. This program collects real-time sums of contact force recordings. These calculations are based on contact force parameters studied in atrial fibrillation (AF) ablations.1-3 The AutoMark lesions represent force-time integral (FTI) or lesion index (LSI) values that can be set by the operator’s preference. The amount of ablation is represented by the size and color of the circle or sphere on the 3D model. Lesion criteria, such as contact force and spatial stability, can also be set by the user. In addition to the voltage signal, it provides a useful tool to visualize areas that might require further ablation. A pertinent example would be finding a gap in a reconnected pulmonary vein that was previously isolated. An example of AutoMark in pulmonary vein isolation (PVI) is shown in Figure 3.
Initial Experience
We have performed 69 cases thus far using EnSite Precision, of which 35 have been for AF (21 paroxysmal, 14 persistent), 18 for ventricular tachycardia (12 ischemic, 6 focal), 8 for atrial tachycardias, 5 for cavotricuspid isthmus atrial flutter ablations, 2 for AV nodal reentry tachycardias, and 1 for AV reentry tachycardia.
We have found that the system performs well in terms of automatic collection of anatomic, voltage, and activation points. Importantly, significant map shift has not been a major problem with this system. This improvement appears to be associated with smaller hydrogel adhesive skin patches as well as the integration of magnetic and impedance mapping. Although surface electrodes can be used as reference points, we have found that coronary sinus electrograms continue to be very useful. The exact location of the coronary sinus catheter can be continually monitored relative to the baseline location throughout the case.
Finally, we have found AutoMark very useful in providing real-time visual feedback, which helps to record consistent lesion creation. This is extremely helpful for pre-selected lesion parameters. We have found these features to be useful and to deliver consistently, both within long complex cases as well as across a broad spectrum of cases.
Our first experience of mapping using EnSite Precision in North America has been promising. This high-quality diagnostic data affords better informed diagnosis and individualized cardiac rhythm management for each patient.
Disclosures: Drs. Redfearn and Glover report consulting fees with Abbott. The other authors have no conflicts of interest to report regarding the content herein.
References
- Neuzil P, Reddy VY, Kautzner J, et al. Electrical reconnection after pulmonary vein isolation is contingent on contact force during initial treatment: results from the EFFICAS I study. Circ Arrhythm Electrophysiol. 2013;6(2):327-333.
- Kautzner J, Neuzil P, Lambert H, et al. EFFICAS II: optimization of catheter contact force improves outcome of pulmonary vein isolation for paroxysmal atrial fibrillation. Europace. 2015;17(8):1229-1235.
- Reddy VY, Dukkipati SR, Neuzil P, et al. Randomized, Controlled Trial of the Safety and Effectiveness of a Contact Force-Sensing Irrigated Catheter for Ablation of Paroxysmal Atrial Fibrillation: Results of the TactiCath Contact Force Ablation Catheter Study for Atrial Fibrillation (TOCCASTAR) Study. Circulation. 2015;132(10):907-915.











