Externalized Conductor of a St. Jude Medical Riata Defibrillator Lead: Case Report and Review of the Literature
Case Report
 A 76-year-old man presented with a medical history of hypertension, dilated cardiomyopathy, coronary artery disease, coronary artery bypass graft surgery, ventricular tachycardia, pericardial effusion, aortic valve replacement, diabetes, and NYHA class II-III congestive heart failure. An implantable cardioverter-defibrillator (ICD) was implanted in 2008 with a St. Jude Medical Riata Model 7000 lead. The patient had underlying atrial flutter with an adequate escape rhythm, and device parameters were: battery voltage 2.42V, atrial amplitude 2.7mV, ventricular amplitude (n/a: patient was pacemaker-dependent), pacing lead impedance 390 ohms (atrial lead) and 430 ohms (ventricular lead), and right ventricular (RV) threshold 0.75V at 0.5 ms. Device reached elective replacement indicator and was referred for revision or replacement.
A 76-year-old man presented with a medical history of hypertension, dilated cardiomyopathy, coronary artery disease, coronary artery bypass graft surgery, ventricular tachycardia, pericardial effusion, aortic valve replacement, diabetes, and NYHA class II-III congestive heart failure. An implantable cardioverter-defibrillator (ICD) was implanted in 2008 with a St. Jude Medical Riata Model 7000 lead. The patient had underlying atrial flutter with an adequate escape rhythm, and device parameters were: battery voltage 2.42V, atrial amplitude 2.7mV, ventricular amplitude (n/a: patient was pacemaker-dependent), pacing lead impedance 390 ohms (atrial lead) and 430 ohms (ventricular lead), and right ventricular (RV) threshold 0.75V at 0.5 ms. Device reached elective replacement indicator and was referred for revision or replacement.
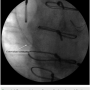
The old RV lead was fluoroscopically examined, and revealed an externalized conductor in the heel of the ICD lead (Figure 1). A new RV lead with a new generator was subsequently implanted.
Discussion
On November 28, 2011, St. Jude Medical released a physician advisory notice about a recall of Riata and Riata ST silicone-insulated leads. Models included in the notice were the Riata 1560-1562, 1570-1572, 1580-1582, and 1590-1592, and Riata ST models 7000-7002, 7010, 7011, and 7040-7042.1 The U.S. Food and Drug Administration (FDA) released a safety communication in regards to the St. Jude Medical advisory.2 The FDA recommendation was to closely monitor patients with recalled leads, reprogram the devices as suggested by the manufacturer, and use fluoroscopic imaging to detect insulation breach. The FDA, St. Jude Medical, and the Heart Rhythm Society do not recommend routine extraction of Riata leads, due to the risks of explantation surgery, and state that removal should be considered in cases of insulation failure observed in imaging in addition to some evidence of abnormal electrical functioning.
In our patient’s case, the decision to add a lead was a clinical one, based on the degree of externalization and the ease of vascular access. A complete extraction was not performed — only a removal of the proximal component of the lead in order to prevent reuse. The lead was therefore cut and capped off.
Numerous studies have evaluated St. Jude Medical’s Riata leads for ICD therapy. These studies have shown lead-related adverse events, including conductor fracture, insulation damage, dislodgement, and perforation. These adverse events sometimes require lead revision, extraction, and/or replacement. The degree of externalization is time-dependent and often includes the lead segment below the tricuspid annulus. Fluoroscopic screening has been performed to evaluate externalization of the recalled Riata leads, and has been demonstrated to have positive and negative predictive value (88 percent and 99 percent, respectively).3
The time-dependent risk to externalization appears to be four to five years’ post-implant.3-5 Hauser and colleagues link 22 deaths to Riata/Riata ST failure from short circuits, but no deaths were linked to conductor externalization.6 A more recent study has shown that postero-anterior and lateral chest X-rays could also be an acceptable mode of imaging for the identification of insulation breaches.7 A separate yet potentially confounding issue is the increased incidence in abrasions against pulse generators linked to Optim-coated St. Jude Medical defibrillator leads, which is beginning to appear in the literature.8 A recent retrospective analysis indicates that the continued use of dual coil leads should be discouraged as they are associated with higher complication rates.9
Conclusions
There is no consensus on how to proceed when a lead is suspected to be defective. Implanted devices ought to be interrogated, but it is not entirely clear whether fluoroscopic screening should be performed. At present, with the recalled Riata lead, the FDA calls for fluoroscopy. However, fluoroscopic evaluation of externalization may not necessarily correlate with lead electrical failure.2-5 Prophylactic replacement should be weighed against the risks of surgery. Further study of the effect of externalization on lead failure and mortality studies is warranted.
Disclosure: Drs. Patel, Amico, Benabou and Cohen have no conflicts of interest to report.
References
- St. Jude Medical Medical Device Advisory. November 28, 2011. Available online at https://www.sjmprofessional.com/Resources/product-notices-advisories/~/media/Cardiac%20Pro/Reference%20and%20Resources/Communications/Nov_2011_Important_Product_Information_Update_Riata_112811.ashx. Accessed March 19, 2013.
- U.S. Food and Drug Administration. FDA Safety Communication: Premature Insulation Failure in Recalled Riata Implantable Cardioverter Defibrillator (ICD) Leads Manufactured by St. Jude Medical, Inc. November 20, 2012. Available online at https://www.fda.gov/MedicalDevices/Safety/AlertsandNotices/ucm314930.htm. Accessed March 19, 2013.
- Larsen JM, Riahi S, Nielsen JC, et al. Nationwide Fluoroscopic Screening of Recalled Riata Defibrillator Leads in Denmark. Heart Rhythm. 2013 Feb 13. pii: S1547-5271(13)00117-3
- Liu J, Rattan R, Adelstein E, et al. Fluoroscopic screening of asymptomatic patients implanted with the recalled Riata lead family. Circ Arrhythm Electrophysiol. 2012;5(4):809-814.
- Theuns DA, Elvan A, de Voogt W, de Cock CC, van Erven L, Meine M. Prevalence and presentation of externalized conductors and electrical abnormalities in Riata defibrillator leads after fluoroscopic screening: report from the Netherlands Heart Rhythm Association Device Advisory Committee. Circ Arrhythm Electrophysiol. 2012;5(6):1059-1063.
- Hauser RG, Abdelhadi R, McGriff D, Retel LK. Deaths caused by the failure of Riata and Riata ST implantable cardioverter-defibrillator leads. Heart Rhythm. 2012;9(8):1227-1235.
- Steinberg C, Sarrazin JF, Philippon F, et al. Detection of high incidence of Riata lead breaches by systematic postero-anterior and lateral chest X-ray in a large cohort. Europace. 2013;15(3):402-408.
- Hauser RG, Abdelhadi RH, McGriff DM, Kallinen Retell L. Failure of a novel silicone-polyurethane copolymer (Optim™) to prevent implantable cardioverter-defibrillator lead insulation abrasions. Europace. 2013;15(2):278-283.
- Epstein LM, Love CJ, Wilkoff BL, et al. Superior vena cava defibrillator coils make transvenous lead extraction more challenging and riskier. J Am Coll Cardiol. 2013;61(9):987-989.
- Epstein AE, Baker JH 2nd, Beau SL, Deering TF, Greenberg SM, Goldman DS. Performance of the St. Jude Medical Riata leads. Heart Rhythm. 2009;6(2):204-209.











