ADVERTISEMENT
Cryoballoon Ablation at the Mayo Clinic
Atrial fibrillation is the most commonly encountered arrhythmia in clinical medicine. The presence of atrial fibrillation is associated with an increased risk of stroke, and patients may complain of palpitations, shortness of breath, and fatigue. Buoyed by the success of ablation for paroxysmal supraventricular arrhythmias and atrial flutter, over the last 15 years investigators from around the world have focused on developing catheter-based ablation techniques for the treatment of atrial fibrillation. At our medical center, atrial fibrillation now accounts for more than 80% of our ablation volume.
As has been emphasized by the 2007 Heart Rhythm Society Guidelines on ablation for atrial fibrillation, isolation of the pulmonary veins remains the “cornerstone” for all ablation procedures for atrial fibrillation.1 Isolation of the pulmonary veins has generally been achieved by placing a series of ablation lesions in the left atrium around the pulmonary veins in a point-by-point fashion using radiofrequency (RF) energy. Although this process has been facilitated by the advent of new mapping tools and irrigated tip ablation catheters, the process can be time consuming and requires even the talented operator to perform a number of procedures to become comfortable. In an effort to shorten the procedure and the “learning curve” for isolating the pulmonary veins, multiple manufacturers have developed catheters designed to produce circular lesions. The first such catheter to be commercially approved in the United States uses cryoenergy delivered by a balloon-based system (Arctic Front®, Medtronic, Minneapolis, MN). Since its approval by the Food and Drug Administration one year ago, over 5,000 cases have been performed in the United States, and worldwide, since its introduction in Europe six years ago, over 25,000 cases have been performed.
Cryoablation
Cryoenergy has been used for almost 50 years for the surgical treatment of arrhythmias and for more than a decade with percutaneous endovascular catheters. Lesions formed by cryoenergy are characterized by relatively sharp borders, dense fibrosis within the lesion with preservation of intercellular architecture, and less disruption of the endovascular surface. Cryoablation causes tissue destruction by the freezing/rewarming cycle, injury to the micro circulation, and triggering of apoptosis (cell death). Although decreased platelet activation and a reduced inflammatory response has been noted with cryoenergy point ablation, a recent study suggests that the systemic inflammatory response is similar for RF catheter ablation and balloon cryoablation.2
The currently available catheter design uses a double balloon system in which nitrous oxide is delivered to an inner balloon through a lumen within the catheter, where it undergoes transformation from liquid to gas producing a temperature of -80°. A thermocouple that measures temperature is located on the proximal portion of the balloon. The catheter has a central lumen for a guidewire (to facilitate placement and reduce the risk of perforation) and to allow for contrast injections to evaluate the seal of the balloon at the pulmonary vein os. Although the catheter is deflectable, a steerable sheath (FlexCath, Medtronic) is used to optimize placement of the balloon within the vein. Optimal balloon placement is critical. Early in development of the cryoballoon, it was recognized that a circumferential seal without periballoon leaks was required to achieve adequate temperatures and produce a fully circular ablation lesion.
Clinical Studies
Between 2000 and 2005, reports from single centers demonstrated the effectiveness of cryoballoon therapy for atrial fibrillation. In the pivotal Sustained Treatment of Paroxysmal Atrial Fibrillation (STOP-AF) Trial, 245 patients with drug refractory atrial fibrillation were randomized to antiarrhythmic drug therapy or cryoballoon ablation.3 After one-year follow-up, 70% of patients treated with cryoablation were free of atrial fibrillation compared to only 7% in the drug therapy arm.
Complications occur in any cardiac procedure, particularly in a complex procedure such as atrial fibrillation ablation. In the STOP-AF Trial, stroke and transient ischemic attacks were observed in 2.5% and 1.8% of patients, respectively. Pulmonary vein stenosis was observed in 3.1% of patients. Phrenic nerve injury was observed in 11.2% of patients. Fortunately, the phrenic nerve injury generally resolved (although taking up to a year in some cases), and in many cases was asymptomatic. In addition to phrenic nerve injury, cryoballoon ablation has been associated with bronchial ulceration. Although esophageal atrial fistula has not been formally reported in the literature, cryoballoon ablation leads to measurable decreases in esophageal luminal temperature and can cause injury to the nerves around the esophagus, producing gastroparesis, and within the last month there have been two preliminary reports of possible esophageal atrial fistula after cryoballoon ablation. Initial reports suggest that atypical atrial flutter is uncommon after cryoballoon ablation. Some investigators have hypothesized that atypical atrial flutters are less likely after cryoablation because the lesions are well demarcated and less likely to have small gaps. Although an appealing explanation, no clinical studies have specifically explored this question. Two studies have evaluated the relationship of ablation technique and cerebral emboli; one found a reduced incidence of cerebral emboli with Arctic Front ablation compared to irrigated RF ablation (cryoablation: 4.3% vs. irrigated RF: 7.4%), while another found no difference between the two techniques (cryoablation: 8.9% vs. irrigated RF: 6.8%).4,5
Comparisons between cryoballoon ablation and RF catheter ablation in nonrandomized cohorts have generally shown the two procedures have similar success rates and complication rates, but cryoballoon ablation has shorter procedure times and fluoroscopy times.6 In patients who require repeat procedures after cryoballoon ablation procedures, recurrent conduction is most commonly observed in the most inferior portion of the vein, which is the most common site for poor contact with the balloon catheter. The FREEZE AF trial is an ongoing multicenter study sponsored by Medtronic and St. Jude Medical that is designed to compare safety and efficacy of cryoballoon ablation and RF catheter ablation using an open irrigated system. The study is in the process of enrolling 244 patients with drug refractory atrial fibrillation who will be randomized to one of the two ablation techniques. The primary endpoint will be freedom from atrial fibrillation off antiarrhythmic medications.
Technical Issues
 Although experienced electrophysiologists and laboratories can easily adopt cryoballoon ablation, there are some technical differences when using this system. Some techniques/tips that we have adopted are summarized in Table 1. We do not change our standard anticoagulation protocol for cryoballoon procedures. Anticoagulation is continued throughout the periprocedural period, and during the procedure we supplement the baseline anticoagulation with additional heparin to maintain the activated clotting time greater than 350 seconds. We acknowledge that this aggressive anticoagulation scheme may increase the risk of bleeding, but in our experience, we have had a minor bleeding complication rate of 3% without major postprocedure bleeding complications such as requirement for transfusion.
Although experienced electrophysiologists and laboratories can easily adopt cryoballoon ablation, there are some technical differences when using this system. Some techniques/tips that we have adopted are summarized in Table 1. We do not change our standard anticoagulation protocol for cryoballoon procedures. Anticoagulation is continued throughout the periprocedural period, and during the procedure we supplement the baseline anticoagulation with additional heparin to maintain the activated clotting time greater than 350 seconds. We acknowledge that this aggressive anticoagulation scheme may increase the risk of bleeding, but in our experience, we have had a minor bleeding complication rate of 3% without major postprocedure bleeding complications such as requirement for transfusion.
Although attention to sheath management is critical for any vascular procedure, it is particularly important with cryoballoon ablation, since air may be present within the folds of the balloon as the catheter is being inserted and within the proximal portion of the FlexCath sheath handle. For these reasons we: 1) aspirate the sheath when the balloon is first introduced, 2) avoid catheter exchanges once the balloon is placed within the heart, 3) minimize the “dwell” time of the sheath within the left atrium by keeping the sheath tip within the right atrium whenever possible (particularly when the sheath is being flushed), and 4) use a continuous infusion of heparinized saline through the sideport of the sheath.
Although double transseptal access provides the advantage of allowing evaluation of pulmonary vein electrograms between occlusions, iatrogenic creation of a persistent atrial septal defect is a possible concern given the size of the FlexCath sheath (outside diameter 15 French). We have adopted a more empiric approach, and will use single transseptal access, performing 3–4 applications to each pulmonary vein in sequential fashion (usually left superior, left inferior, right superior, and right inferior pulmonary veins) without evaluating pulmonary vein/left atrial conduction between ablations. A specialized 3.3 Fr circular mapping catheter with eight electrodes (Achieve, Medtronic) that can be deployed through the lumen of the cryoballoon catheter has recently been released for commercial use.
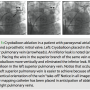 Lower temperatures correlate with a higher likelihood of an effective ablation and are achieved if the vein can be completely occluded. During cryoballoon ablation, temperature is continuously monitored; there is a rapid decrease in the temperature during the first 30 seconds that begins to plateau between 60–90 seconds, with the coldest temperatures achieved after approximately 180 seconds. At 90 seconds, the average temperature that produces isolation is -45° for the superior veins and -40° for inferior veins, but significant overlap exists between effective and ineffective ablations. In practice, we try to achieve temperatures < -45° at 90 seconds, but will accept lower temperatures for inferior veins. Although some amount of forward pressure is necessary to achieve an initial “seal,” it is important for the operator to remember that sometimes too much pressure will exacerbate an inferior leak, particularly if the steerable sheath has a significant curve. As shown in Figure 1, although the cryoballoon catheter is steerable, usually the most effective way for achieving occlusion is to change the orientation of the balloon by wiring different branches of the pulmonary vein. We initially evaluate the angle of the “take-off” in both the left anterior oblique and right anterior oblique orientations and first try to position the shaft of the catheter in the center of the pulmonary vein ostium at the same angle as the pulmonary vein “take-off.” Although this is a reasonable initial strategy, it is important to remember that there are no “hard and fast” rules, and with experience the operator will find the best solution for an individual patient. The inferior border is the most common site for “leaks,” and reconnection of pulmonary veins is most common in the inferior part of the ostia. Not surprisingly, atypical pulmonary venous anatomy, such as a common antrum, is more likely to be associated with requirement for repeat procedures.
Lower temperatures correlate with a higher likelihood of an effective ablation and are achieved if the vein can be completely occluded. During cryoballoon ablation, temperature is continuously monitored; there is a rapid decrease in the temperature during the first 30 seconds that begins to plateau between 60–90 seconds, with the coldest temperatures achieved after approximately 180 seconds. At 90 seconds, the average temperature that produces isolation is -45° for the superior veins and -40° for inferior veins, but significant overlap exists between effective and ineffective ablations. In practice, we try to achieve temperatures < -45° at 90 seconds, but will accept lower temperatures for inferior veins. Although some amount of forward pressure is necessary to achieve an initial “seal,” it is important for the operator to remember that sometimes too much pressure will exacerbate an inferior leak, particularly if the steerable sheath has a significant curve. As shown in Figure 1, although the cryoballoon catheter is steerable, usually the most effective way for achieving occlusion is to change the orientation of the balloon by wiring different branches of the pulmonary vein. We initially evaluate the angle of the “take-off” in both the left anterior oblique and right anterior oblique orientations and first try to position the shaft of the catheter in the center of the pulmonary vein ostium at the same angle as the pulmonary vein “take-off.” Although this is a reasonable initial strategy, it is important to remember that there are no “hard and fast” rules, and with experience the operator will find the best solution for an individual patient. The inferior border is the most common site for “leaks,” and reconnection of pulmonary veins is most common in the inferior part of the ostia. Not surprisingly, atypical pulmonary venous anatomy, such as a common antrum, is more likely to be associated with requirement for repeat procedures.
To monitor for phrenic nerve paralysis, we use a circular mapping catheter and pace from a widely spread bipole that encompasses one quadrant of the circumference (e.g., pacing from electrodes 1 and 5) at high outputs (20 mA). With rotation of the catheter and movement up and down the superior vena cava, it is extremely easy to find a site with stable phrenic nerve pacing. It is important to monitor the strength of diaphragmatic excursion during ablation and to stop the ablation once any changes are identified. Phrenic nerve injury usually occurs during ablation of the right superior pulmonary vein, but in practice, we pace during any ablations performed on the right-sided pulmonary veins. Since phrenic nerve injury is more likely with smaller balloons (23 mm), we, like other centers, use the larger balloon (28 mm) exclusively. Using this method we have not had any cases of phrenic nerve paralysis, although in approximately 1 of every 15–20 patients, we will not be able to use the cryoballoon for the right superior pulmonary vein.
Generally, if any regions of continued pulmonary vein conduction remain after cryoballoon ablation, they are quite small. For this reason, once we have performed cryoballoon ablations in all four pulmonary veins, we will remove the cryoballoon and generally not use it for the rest of the case. Any residual areas of pulmonary vein conduction are ablated with RF using conventional methods. The septal defect created by the 15 Fr sheath required for cryoballoon ablation can easily accommodate two catheters (ablation and mapping catheters). With this workflow, we only insert the cryoballoon catheter once with a single transseptal access.
 Finally, it is important to have a dedicated staff that is committed to cryoballoon ablation since the technical aspects are different than radiofrequency catheter ablation (Figure 2). Four hands at the table facilitate cryoballoon placement at the pulmonary vein os: two hands for manipulating the catheter and sheath, and two hands for providing contrast, pressure monitoring, and guidewire management at the hemostatic valve. We have a third person at the stimulator for phrenic nerve pacing. Post-hoc analysis of the STOP-AF trial shows the important effect of experience for both the operator (and the center).7 At experienced centers, success rates were higher (88% vs. 67%), procedure times were shorter (317 minutes vs. 371 minutes, fluoroscopy times were shorter (32 minutes vs. 63 minutes), and there was a decrease in phrenic nerve injury (5.6% vs. 11.2% in the original cohort).
Finally, it is important to have a dedicated staff that is committed to cryoballoon ablation since the technical aspects are different than radiofrequency catheter ablation (Figure 2). Four hands at the table facilitate cryoballoon placement at the pulmonary vein os: two hands for manipulating the catheter and sheath, and two hands for providing contrast, pressure monitoring, and guidewire management at the hemostatic valve. We have a third person at the stimulator for phrenic nerve pacing. Post-hoc analysis of the STOP-AF trial shows the important effect of experience for both the operator (and the center).7 At experienced centers, success rates were higher (88% vs. 67%), procedure times were shorter (317 minutes vs. 371 minutes, fluoroscopy times were shorter (32 minutes vs. 63 minutes), and there was a decrease in phrenic nerve injury (5.6% vs. 11.2% in the original cohort).
Final Thoughts
At our facility, we use all of the commercially available methods for mapping of atrial fibrillation and catheter-based ablation. I tend to recommend cryoballoon ablation for a first procedure in a patient with paroxysmal atrial fibrillation. In patients with paroxysmal atrial fibrillation, additional ablation within the atria is generally not required and bleeding complications from the large sheath are more manageable since protamine sulfate often can be used to reverse the effects of intraprocedural heparin. In patients who have had prior ablation for atrial fibrillation, often much of the pulmonary vein/left atrial junction has been ablated and the circumferential lesion produced by the cryoballoon catheter is not necessary. Although combined procedures using both cryoballoon ablation and RF ablation for persistent atrial fibrillation have been reported, this is generally not our laboratory’s practice. Since 30–50 cc of contrast is usually required through the procedure to confirm balloon placement, renal function should be normal. In reviewing our cases for the past year, cryoballoon ablation shortens procedure time by 30–35% and left atrial “dwell” time is shortened by 25% when compared to RF ablation.
It is important to remember that we are using the first generation of balloon technology. The next-generation catheters will utilize conforming balloons that will more reliably occlude vessels, even when atypical pulmonary venous anatomy is present. Other catheter designs such as laser balloons and multiple electrode catheters that produce multiple lesions with single applications are already being used outside the United States or are in development. We are at the cusp of a new era of ablation for arrhythmias similar to the transition 25 years ago when RF supplanted direct current energy.
Acknowledgement. Thanks to Tammy Pullen for reviewing the final manuscript.
References
- European Heart Rhythm Association (EHRA); European Cardiac Arrhythmia Society (ECAS); American College of Cardiology (ACC); American Heart Association (AHA); Society of Thoracic Surgeons (STS), Calkins H, Brugada J, Packer DL, et al. HRS/EHRA/ECAS expert Consensus Statement on catheter and surgical ablation of atrial fibrillation: Recommendations for personnel, policy, procedures and follow-up. A report of the Heart Rhythm Society (HRS) Task Force on catheter and surgical ablation of atrial fibrillation. Heart Rhythm 2007;4:816–861.
- Herrera Siklódy C, Arentz T, Minners J, et al. Cellular damage, platelet activation, and inflammatory response after pulmonary vein isolation: A randomized study comparing radiofrequency ablation with cryoablation. Heart Rhythm 2011 Sept 13. [Epub ahead of print]
- Packer DH. STOP AF (Sustained Treatment of Paroxysmal Atrial Fibrillation) Trial presented at the late-breaking sessions at the 59th Annual Scientific Session of the American College of Cardiology in Atlanta.
- Herrera Siklódy C, Deneke T, Hocini M, et al. Incidence of asymptomatic intracranial embolic events after pulmonary vein isolation: Comparison of different atrial fibrillation ablation technologies in a multicenter study. J Am Coll Cardiol 2011;58:681–688.
- Neumann T, Kuniss M, Conradi G, et al. MEDAFI-Trial (Micro-embolization during ablation of atrial fibrillation): Comparison of pulmonary vein isolation using cryoballoon technique vs. radiofrequency energy. Europace 2011;13:37–44.
- Kojodjojo P, O’Neill MD, Lim PB, et al. Pulmonary venous isolation by antral ablation with a large cryoballoon for treatment of paroxysmal and persistent atrial fibrillation: Medium-term outcomes and non-randomised comparison with pulmonary venous isolation by radiofrequency ablation. Heart 2010;96:1379–1384.
- Packer DH, Kowal RC, Wheelan KR, et al. Impact of experience on efficacy and safety of cryoballoon ablation for atrial fibrillation: Outcomes of the STOP-AF Continued Access Protocol. Heart Rhythm 2011; S379.











