Contact Force Mapping with Remote Manipulation of a Mapping Catheter
Introduction
Several systems have been developed and approved by the United States Food and Drug Administration (FDA) for remote catheter manipulation during electrophysiology procedures. These include systems by Stereotaxis, Inc., Hansen Medical, and Catheter Robotics. The Stereotaxis Niobe® system is a magnetically driven robotic system that requires a large construction footprint to encompass two large steerable magnets and a separate computerized control room. Hansen Medical’s Sensei® X Robotic System uses a mechanically driven device employing a large steerable sheath and console/interface. The Catheter Robotics Amigo™ system uses a robotic manipulator to control standard sheaths and catheters, together with a simple small remote handle controller in order to mechanically manipulate the catheter.1 Perhaps because of its relatively low cost, the last system has recently drawn attention.
The above systems have limitations based on the contact force made between the catheter tip and the myocardium. Niobe® may be the safest, even without contact force sensing, since a magnetic field pulls the catheter tip gingerly towards the myocardium. It is conceivable that in some of their procedures, contact force may not be adequate for effective ablative lesions. On the contrary, mechanical systems (Sensei® and Amigo™), in which the catheter is mechanically driven against the myocardium, may produce untoward risk of potential myocardial damage and perforation. In order to minimize this risk, Hansen Medical has developed a system called IntelliSense® to sense contact force by placing force sensors in the tip of their sheath.2 Until recently, the Catheter Robotics system had no mechanism, other than visual fluoroscopic feedback and the size of the electrogram signal, to evaluate endocardial contact.
Recently, Biosense Webster, Inc., a Johnson and Johnson company, received FDA approval of their contact force-sensing catheter (ThermoCool® SmartTouch™). This catheter integrates contact force sensing into their Carto® 3 non-fluoroscopic mapping system. This magnetic-based technology precisely monitors contact force during mapping procedures.8 Here we describe a case report of remote mapping with the Amigo™ Remote Catheter System using the Carto® 3 System and SmartTouch™ catheter.
Case Report
A 63-year-old female patient with history of pacemaker implantation, supraventricular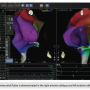 tachycardia, nonsustained ventricular tachycardia, and palpitations was referred to our institution for electrophysiology study and possible ablation with robotic assistance. The patient’s baseline conduction was within normal limits. Atrial flutter was induced along with atrial fibrillation. The patient underwent remote mapping with the Amigo™ and Carto® 3 System and SmartTouch™. Although the patient had no inducible ventricular tachycardia, she did have sustained clockwise atrial flutter, which was mapped with the Carto® 3 System. Figure 1 demonstrates clockwise atrial flutter in the right anterior oblique and left anterior oblique. Figure 2 demonstrates the post-ablation map, showing complete isthmus block during coronary sinus pacing following radiofrequency catheter ablation of the cavotricuspid isthmus
tachycardia, nonsustained ventricular tachycardia, and palpitations was referred to our institution for electrophysiology study and possible ablation with robotic assistance. The patient’s baseline conduction was within normal limits. Atrial flutter was induced along with atrial fibrillation. The patient underwent remote mapping with the Amigo™ and Carto® 3 System and SmartTouch™. Although the patient had no inducible ventricular tachycardia, she did have sustained clockwise atrial flutter, which was mapped with the Carto® 3 System. Figure 1 demonstrates clockwise atrial flutter in the right anterior oblique and left anterior oblique. Figure 2 demonstrates the post-ablation map, showing complete isthmus block during coronary sinus pacing following radiofrequency catheter ablation of the cavotricuspid isthmus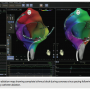 with the ThermoCool® SmartTouch™ catheter. This irrigated tip catheter uses a precision spring system, which records microdeformations in order to track contact force and assure safe and appropriate myocardial contact to optimize the ablation procedure.7 This system was quite helpful at permitting real-time monitoring of contact force during remote mapping, which was done entirely from the control room. Figure 3 shows the operator holding the Amigo™ used in the procedure, along with the Carto® 3 System from the control room. Contact force sensing with the Carto® 3 System and SmartTouch™ appeared to provide an additional layer of feedback during the remote catheter manipulation procedure.
with the ThermoCool® SmartTouch™ catheter. This irrigated tip catheter uses a precision spring system, which records microdeformations in order to track contact force and assure safe and appropriate myocardial contact to optimize the ablation procedure.7 This system was quite helpful at permitting real-time monitoring of contact force during remote mapping, which was done entirely from the control room. Figure 3 shows the operator holding the Amigo™ used in the procedure, along with the Carto® 3 System from the control room. Contact force sensing with the Carto® 3 System and SmartTouch™ appeared to provide an additional layer of feedback during the remote catheter manipulation procedure.
Discussion
Contact force sensing using Hansen Medical’s proprietary IntelliSense® system has been previously integrated with robotic/remote catheter manipulation into their Sensei® X Robotic Catheter System. This system uses a 3D joystick (Instinctive Motion Controller) to navigate the catheter.4 However, it requires special steerable sheaths and a rather large computerized console system in order to remotely operate.4 Sterotaxis has no method to augment their contact force with the myocardium. Their system is exceedingly safe; however, it may not apply enough pressure against the myocardium to produce consistent effective ablative lesions. Contact force using more traditional ablation catheters recently was approved by the U.S. Food and Drug Administration for the Carto® SmartTouch™ system. Additionally, Endosense, which was purchased by St. Jude Medical in August 2013, has developed the TactiCath® contact force sensing catheter.5 This is an open-irrigated ablation catheter, much like the SmartTouch™, but uses a triaxial fiberoptic cable inside the catheter to measure contact force.6 The TactiCath® awaits approval by the U.S. FDA.
previously integrated with robotic/remote catheter manipulation into their Sensei® X Robotic Catheter System. This system uses a 3D joystick (Instinctive Motion Controller) to navigate the catheter.4 However, it requires special steerable sheaths and a rather large computerized console system in order to remotely operate.4 Sterotaxis has no method to augment their contact force with the myocardium. Their system is exceedingly safe; however, it may not apply enough pressure against the myocardium to produce consistent effective ablative lesions. Contact force using more traditional ablation catheters recently was approved by the U.S. Food and Drug Administration for the Carto® SmartTouch™ system. Additionally, Endosense, which was purchased by St. Jude Medical in August 2013, has developed the TactiCath® contact force sensing catheter.5 This is an open-irrigated ablation catheter, much like the SmartTouch™, but uses a triaxial fiberoptic cable inside the catheter to measure contact force.6 The TactiCath® awaits approval by the U.S. FDA.
Contact force appears to be useful in assuring the creation of durable ablation lesions. Sacher and colleagues compared the impact of force sensing using TactiCath® to standard ThermoCool® without "SmartTouch™" force sensing (prior to its availability) in an ovine model.6 They observed that in cases without contact force sensing, 22 percent of endocardial radiofrequency ablations that were thought to have good contact failed to produce a durable lesion.6 Reichlin and colleagues compared contact force sensing using both the SmartTouch™ and the TactiCath® during atrial fibrillation ablation.7 They observed that good contact force was associated with a decrease in initial impedance, which led to a longer lasting lesion.7 Remote/robotic mapping systems have the ability to lower fluoroscopy exposure to operators, and could also potentially lower radiation exposure to the patient. Thomas and colleagues found that fluoroscopy time was reduced by 26 percent using the Sensei® X.8
In this case report, we used the ThermoCool® SmartTouch™ catheter with contact force sensing together with the Amigo™ Robotic Catheter System. These two devices may complement one another and permit remote catheter manipulation with continuous contact force feedback. Additional experience is required before one can determine the true utility of this dual manufacturer/integrated technology.
Disclosures: The authors have no conflicts of interest to report regarding the content herein. Dr. Cohen is the original inventor and is listed on at least 3 issued Catheter Robotics patents. These patents belong to Catheter Robotics and importantly, Dr. Cohen does not receive royalties from them. There is no other relevant information within the past 36 months.
Editor’s Note: This article underwent peer review by one or more members of EP Lab Digest®’s editorial board.
References
- Cohen, Todd J. The Future of Electrophysiology. Practical Electrophysiology, Second Edition. Malvern, Penn.: HMP Communications, 2009. 267-273. Print.
- Di Biase L, Paoletti Perini A, Mohanty P, et al. Visual, tactile, and contact force feedback: which one is more important for catheter ablation? Results from an in vitro experimental study. Heart Rhythm. 2014;11(3):506-513.
- Knight B, Ayers GM, Cohen TJ. Robotic positioning of standard electrophysiology catheters: a novel approach to catheter robotics. J Invasive Cardiol. 2008;20(5):250-253.
- Reddy VY, Neuzil P, Malchano ZJ, et al. View-synchronized robotic image-guided therapy for atrial fibrillation ablation: experimental validation and clinical feasibility. Circulation. 2007;115(21):2705-2714.
- Perriello, Brad. "St. Jude Medical Touches Endosense for $331M." MassDevice Newsletter. The Medical Device Business Journal, 19 Aug. 2013. Web. 13 Aug. 2014.
- Sacher F, Wright M, Derval N, et al. Endocardial versus epicardial ventricular radiofrequency ablation: utility of in vivo contact force assessment. Circ Arrhythm Electrophysiol. 2013;6(1):144-150.
- Reichlin T, Knecht S, Lane C, et al. Initial impedance decrease as an indicator of good catheter contact: insights from radiofrequency ablation with force sensing catheters. Heart Rhythm. 2014;11(2):194-201.
- Thomas D, Scholz EP, Schweizer PA, Katus HA, Becker R. Initial experience with robotic navigation for catheter ablation of paroxysmal and persistent atrial fibrillation. J Electrocardiol. 2012;45(2):95-101.











