A Comparison of the New Anticoagulants for Use in Atrial Fibrillation
Atrial fibrillation (AF) is the most common cardiac rhythm disorder, affecting at least 1% of the general population. After age 80 this increases to 10% of the population.1 In addition, the presence of AF is responsible for a 4- to 5-fold increase in the risk of ischemic stroke.2
Treatment for ischemic stroke prevention in the past has been limited to warfarin, a vitamin K antagonist, and aspirin. Use of warfarin requires ongoing anticoagulant monitoring and resultant dosage adjustments. However, Connolly et al found that even during randomized clinical trials patients were only within warfarin therapeutic range two-thirds of the time.3 Limitations of warfarin therapy include slow onset of action, a narrow therapeutic window, and multiple diet and drug interactions.4 In addition, warfarin use is associated with a risk of bleeding of 2% per year. Aspirin or aspirin with clopidogrel use leads to similar bleeding risk.5 Aspirin has been associated with a small reduction in stroke incidence.6
Recently, the drug dabigatran was approved for stroke prevention in the AF population, followed closely by rivaroxaban. The drug apixaban is also currently undergoing FDA review. These 3 drugs approach anticoagulation via different mechanisms from warfarin, and the clinical trial results for each varies. In addition, therapy with these drugs does not require anticoagulant effect monitoring, and they render their effect at a fixed dose. The following is a summary of these new warfarin rivals.
The Challengers
Dabigatran (Pradaxa, Boehringer Ingelheim)
Dabigatran etexilate, 150 mg twice daily, is a direct thrombin inhibitor, affecting factor IIa of the coagulation cascade (Figure 1). Pradaxa (dabigatran) received FDA approval in October 2010 for patients with non-valvular AF, at a dose of 150 mg twice a day.7
Pharmacokinetics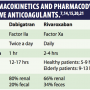 The half-life of the drug is 12 to 17 hours, and it is 80% renally excreted (Table 1).8 Therefore, the drug may accumulate in the setting of abnormal renal function.
The half-life of the drug is 12 to 17 hours, and it is 80% renally excreted (Table 1).8 Therefore, the drug may accumulate in the setting of abnormal renal function.
Dabigatran was studied in a variety of settings: orthopedic, stroke prevention in the AF population, and secondary prevention in acute coronary syndrome.
The RE-LY Trial The pivotal trial for use in the AF population was the RE-LY (Randomized Evaluation of Long-term anticoagulation therapy) trial, a noninferiority study that compared dabigatran to warfarin.9 The goal of this study was stroke prevention, with secondary endpoints of myocardial infarction, pulmonary embolism, hospitalization, total mortality, and cardiovascular mortality. Patients were randomized to 110 or 150 mg of dabigatran b.i.d. or warfarin to maintain the INR at 2.0 to 3.0. The trial enrolled 18,113 AF patients. At the two-year follow-up, the stroke or embolism rate for the 110 mg group was equivalent to warfarin, and it was less often for those taking the 150 mg dose. Hemorrhagic stroke occurred less for both dose levels of dabigatran when compared to warfarin. The rate of major bleeding was comparable for high-dose dabigatran and warfarin but lower for low dose dabigatran (Table 2).
The pivotal trial for use in the AF population was the RE-LY (Randomized Evaluation of Long-term anticoagulation therapy) trial, a noninferiority study that compared dabigatran to warfarin.9 The goal of this study was stroke prevention, with secondary endpoints of myocardial infarction, pulmonary embolism, hospitalization, total mortality, and cardiovascular mortality. Patients were randomized to 110 or 150 mg of dabigatran b.i.d. or warfarin to maintain the INR at 2.0 to 3.0. The trial enrolled 18,113 AF patients. At the two-year follow-up, the stroke or embolism rate for the 110 mg group was equivalent to warfarin, and it was less often for those taking the 150 mg dose. Hemorrhagic stroke occurred less for both dose levels of dabigatran when compared to warfarin. The rate of major bleeding was comparable for high-dose dabigatran and warfarin but lower for low dose dabigatran (Table 2).
Contraindications, Interactions, and Adverse Events
Dabigatran is contraindicated with active pathological bleeding or hypersensitivity.
There were no reported significant rises in liver enzymes in the RE-LY study; there were no interactions with cytochrome P450 enzymes.
At steady-state with therapeutic doses, the drug increases aPTT 1.5-2 times control.
There is an increased risk of bleeding if the patient is on antiplatelet agents, heparin, fibrinolytic therapy, or takes NSAIDs chronically.
Dabigatran may be given with or without food. It should be given ≥ 2 hours before an antacid or proton pump inhibitor, as these drugs may decrease dabigatran concentration. Rifampin may decrease the effects of dabigatran. Dose adjustments are not required with ketoconazole, amiodarone, verapamil, clarithromycin, and quinidine. It is recommended that dabigatran be given ≥ 2 hours before giving these drugs. St. John’s wort may decrease dabigatran concentration.
There is an increased risk of stroke if the drug is discontinued.
Adverse GI effects include dyspepsia, gastritis, and bleeds within the first couple of months; histamine-2 receptor antagonists may help dyspepsia,7 but are prescribed cautiously. There was a higher rate of GI bleeds with dabigatran than with warfarin.7
Monitoring and Reversal
Drug therapy with dabigatran does not require regular monitoring. The level of anticoagulation can be assessed in cases of bleeding by measuring the thrombin clotting time or ecarin clotting time. Activated partial thromboplastin time does not give a totally accurate quantification of the level of anticoagulation, but may help determine if anticoagulation is excessive in an emergency situation.10
There is no antidote. It has been theorized that factor VIIa and activated prothrombin complex concentrates would be possible reversal agents; dialysis is also recommended.10 Once the drug enters the body, there is a low affinity for protein binding. Sixty percent of the drug is removed after 2–3 hours of dialysis. Activated charcoal may be administered within 1–2 hours of overdose, to decrease exposure.7 The best approach is thought to be use of fresh frozen plasma, mechanical compression, and surgical hemostasis.7 Maintenance of adequate diuresis is also important, as the drug is primarily excreted in the urine.
A recently published study looked at the cost-effectiveness of dabigatran compared with warfarin for stroke prevention in atrial fibrillation.11 The outcome measures for this study were quality-adjusted life-years (QALYs), costs, and incremental cost-effectiveness ratios. The study concluded that for the ≥ 65-year-old population at increased risk for stroke, dabigatran may be a cost-effective alternative to warfarin depending on the drug pricing of dabigatran.
Dabigatran has been available now for a little over one year. A few issues related to therapy with the drug have been identified:12
- The cost of dabigatran is ~$7 per day vs. warfarin $0.22 per day. For those who pay out of pocket, this is excessive. For those whose medications are purchased through copay, it is thought the insurers will review the data related to the decreased stroke incidence with this drug and consider the ultimate savings.
- There is a 6% rate of major GI bleeds during each year of treatment with dabigatran plus an increase in the incidence of gastritis; coadministration of antiplatelet drugs may contribute to this, and care providers need to be aware of this.
- There is no antidote.
- Twice a day dosing tends to decrease compliance levels.
- The drug blister pack cannot be opened days in advance due to drug potency loss. This prevents patients from lining up their meds for a week at a time, a maneuver that increases compliance.
A medication guide is available to give to the patient when the medication is prescribed.
Xa inhibitors
Xa inhibition prevents prothrombin from being generated from thrombin, and inhibits the generation of tissue factor-induced thrombin.13 Apixaban, betrixaban, edoxaban, and rivaroxaban are the drugs that inhibit factor Xa (Figure 1). This discussion includes rivaroxaban and apixaban.
Rivaroxaban (Xarelto, Bayer/Johnson & Johnson)
Xarelto (rivaroxaban) was approved by the FDA in November 2011 for use in patients with non-valvular atrial fibrillation. It was approved in July 2011 for use after knee or hip replacement surgery, to reduce the risk of blood clots, DVT, and pulmonary embolism. The FDA has required that patients getting the drug must receive a medication guide describing the risks and adverse reactions associated with the drug. The drug should be taken once a day with the evening meal to ensure complete absorption.
Pharmacokinetics
Rivaroxaban is 80% bioavailable. Maximal plasma concentrations are reached after 3–4 hours14 and rivaroxaban has a plasma half-life of 7 to 11 hours. Two-thirds of the drug is metabolized by the liver; one-third goes unchanged through renal excretion (Table 1).15
The ROCKET AF Study
Rivaroxaban was first studied in the orthopedic population. The ROCKET AF study was conducted from December 2006 to May 2010.16 This was a double-blind trial design in which computer-generated sham INR values were generated for rivaroxaban patients to eliminate treatment bias. The Phase III ROCKET AF study enrolled 14,264 patients and was conducted in 45 countries. Patients with nonvalvular AF and a history of stroke or two additional independent risk factors for stroke were randomized to 20 mg of rivaroxaban daily (15 mg daily if creatinine clearance is 30 to 49 ml/min) or warfarin with the dose adjusted to maintain the INR at 2.0 to 3.0.17 For this study, the primary efficacy endpoint was new stroke or systemic embolism, and the safety endpoint was a major bleeding event or clinically relevant nonmajor bleeding event. Secondary efficacy endpoints were all-cause death, vascular death, and MI.
Rivaroxaban was found to be noninferior to warfarin for stroke or systemic embolism prevention. Major bleeding incidence was equivalent, but intracranial and fatal bleeding occurred less frequently in rivaroxaban (Table 2).
Contraindications, Interactions, and Adverse Events
The drug is not recommended for those receiving ketoconazole, lopinavir/ritonavir, ritonavir, indinavir/ritonavir, conivaptan, HIV protease inhibitors, rifampin, itraconazole, voriconazole, or posaconazole.
Avoid use with moderate and severe hepatic impairment or with any hepatic disease associated with coagulopathy.
There are no reported food-drug interactions and little drug-drug interaction potential.
Phenytoin, phenobarbital, carbamazepine, rifampin, and St. John’s wort may reduce rivaroxaban concentration. Aspirin, clopidogrel, naproxen, erythromycin, fluconazole, and clarithromycin use may increase bleeding. Use anticoagulants, NSAIDs/aspirin, and clopidogrel concomitantly with caution.
If creatinine clearance is between 15–50 mL/min, rivaroxaban should be used with caution with amiodarone, diltiazem, verapamil, chloramphenicol, cimetidine, and erythromycin.
A boxed warning states that sudden discontinuation increases the risk of stroke.
The most common adverse reactions were bleeding complications.
Monitoring and Reversal
In case of overdose, dialysis is not thought to be of benefit. There is no antidote available. However, activated charcoal may be used to reduce absorption.18
Apixaban (Eliquis, Pfizer/Bristol-Myers Squibb)
The FDA will decide on the approval status of apixaban by March 28, 2012.19 A couple of very successful clinical trials increases the chances for this drug’s approval in 2012.
Pharmacokinetics
Apixaban has a rapid onset of action and high oral bioavailability. The half-life is ~12 hours. Food does not affect absorption, and the drug is not altered by pH changes. Elimination is 25% renal and 75% nonrenal (Table 1).20,21
The ARISTOTLE Trial
The ARISTOTLE Trial was the first apixaban study in the AF population. The trial enrolled 18,201 patients with AF with at least one additional risk factor for stroke. The patients were randomized to warfarin with INR maintained between 2 and 3, and apixaban 5 mg twice a day. A lower apixaban dose was given to those: ≥ 80 years old, or body weight ≤ 60 kg, or creatinine 1.5mg/dL (2.5 of apixaban). Primary efficacy for the study was a stroke or systemic embolism or hemorrhagic stroke; primary safety outcome was major bleeding.20,21
Superior efficacy and safety was shown with apixaban compared with warfarin. There was improvement in all-cause mortality. There was also a reduction in CNS events that was slightly less than in the RE-LY trial. There was also reduced major bleeding. The reduced all-cause death significance was p=0.047, the first non-warfarin agent to do better than warfarin (Table 2).
The AVERROES Trial
The AVERROES Trial enrolled 5,599 patients, and compared apixaban to aspirin (ASA). There are patients who are unable to take warfarin who therefore take aspirin as an anticoagulant. This study of AF patients with at least one risk factor for stroke, who have failed or are unable to take warfarin, compared apixaban 5 mg twice a day (2.5 mg for people who satisfy two of these: ≥ 80 years, body weight ≤ 60 kg, serum creatinine ≥ 1.5 mg/dL, or 133 μmol/L) to ASA 81 to 324 mg per day. For this study, stroke or systemic embolism were the primary efficacy outcomes, while major bleeding was the primary safety outcome. The trial was halted prematurely as there was a clear benefit in favor of apixaban.22,23
The results reported included a reduced risk of stroke and systemic embolism; no increased risk of major bleeding or intracranial hemorrhage was seen. There were significantly fewer all-cause deaths, and the risk of first hospitalization was significantly reduced.
Contraindications, Interactions, and Adverse Events
Apixaban is not to be taken in combination with inhibitors of CYP3A4 (azole antifungals, macrolide antibiotics, protease inhibitors); these agents increase apixaban levels.24
Complete data regarding interactions and adverse events is not yet available.
Monitoring and Reversal
Disadvantages of the drug are the inability to monitor and the twice a day dosing.25 As a result, adherence cannot be tested. The rapid onset of action could be both an advantage and a disadvantage. Also, there is no antidote available.
The Antidote Issue
A letter to the editor of the New England Journal of Medicine in November discussed the dilemma of caring for trauma patients who were on dabigatran. Several patients who had been treated died due to bleeding that was not reversible, as there are no antidotes to dabigatran (which is also the case with the factor Xa inhibitors).26
There are attempts being made to remedy this situation. A factor Xa inhibitor antidote is currently being studied by Portola Pharmaceuticals.27 In August 2011, Portola presented data from an animal study in which they achieved reversal of rivaroxaban-mediated anticoagulation with PRT064445, the possible antidote being developed.28 There is also work being done to develop a factor VIIa prothrombin complex concentrate and activated prothrombin complex for possible reversal of dabigatran anticoagulation.29
Summary
The introduction of new options for anticoagulation in AF patients is a welcome development. The fact that laboratory monitoring is not required and fixed dosing is possible are added benefits. However, antidote development for these drugs is crucial. I think we all look forward to what comes next.
References
- Go AS, Hylek EM, Phillips KA, et al. Prevalence of diagnosed atrial fibrillation in adults: National implications for rhythm management and stroke prevention: the AnTicoagulation and Risk Factors In Atrial Fibrillation (ATRIA) study. JAMA 2001;285:2370–2375.
- Wolf PA, Abbott RD, Kannel WB. Atrial fibrillation as an independent risk factor for stroke: The Framingham Study. Stroke 1991;22:983–988.
- Connolly SJ, Pogue J, Eikelboom J, et al. ACTIVE W Investigators. Benefit of oral anticoagulant over antiplatelet therapy in atrial fibrillation depends on the quality of international normalized ratio control achieved by centers and countries as measured by time in therapeutic range. Circulation 2008;118:2029–2037.
- Eikelboom JW, Weitz JI. A replacement for warfarin: the search continues. Circulation 2007;116:131–133.
- Connolly S, Pogue J, Hart R, et al. Clopidogrel plus aspirin versus oral anticoagulation for atrial fibrillation in the Atrial fibrillation Clopidogrel Trial with Irbesartan for prevention of Vascular Events (ACTIVE W): a randomised controlled trial. Lancet 2006;367:1903–1912.
- Hart RG, Pearce LA, Aguilar MI. Meta-analysis: antithrombotic therapy to prevent stroke in patients who have nonvalvular atrial fibrillation. Ann Intern Med 2007;146:857–867.
- Boehringer Ingelheim Pharmaceuticals, Inc. Pradaxa (dabigatran) package insert. Ridgefield, CT; 2011.
- Stangier J. Clinical pharmacokinetics and pharmacodynamics of the oral direct thrombin inhibitor dabigatran etexilate. Clin Pharmacokinet 2008;47:285–295.
- Connolly SJ, Ezekowitz MD, Yusuf S, et al. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med 2009;361:1139–1151.
- van Ryn J, Stangier J, Haertter S, et al. Dabigatran etexilate — a novel, reversible, oral direct thrombin inhibitor: interpretation of coagulation assays and reversal of anticoagulant activity. Thromb Haemost 2010;103:1116–1127.
- Freeman JV, Zhu RP, Owens DK, et al. Cost-effectiveness of dabigatran compared with warfarin for stroke prevention in atrial fibrillation. Ann Intern Med 2011;142:1–11.
- Zoler ML. Dabigatran’s First Atrial Fib Year Syarts Warfarin’s Decline. Cardiology News 2011;9:1,8.
- Gerotziafas GT, Elalamy I, Depasse F, et al. In vitro inhibition of thrombin generation, after tissue factor pathway activation, by the oral, direct factor Xa inhibitor rivaroxaban. J Thromb Haemost 2007;5:886–888.
- Kubitza D, Becka M, Wensing G, et al. Safety, pharmacodynamics, and pharmacokinetics of BAY 59-7939 — an oral, direct Factor Xa inhibitor — after multiple dosing in healthy male subjects. Eur J Clin Pharmacol 2005;61:873–880.
- Kubitza D, Becka M, Mueck W, et al. Safety, tolerability, pharmacodynamics, and pharmacokinetics of rivaroxaban — an oral, direct factor Xa inhibitor — are not affected by aspirin. J Clin Pharmacol 2006;46:981–990.
- Patel MR, Mahaffey KW, Garg J, et al. Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med 2011;365:883–891.
- Executive Steering Committee, on behalf of the ROCKET AF Study Investigators. Rivaroxaban-once daily, oral, direct factor Xa inhibition compared with vitamin K antagonism for prevention of stroke and embolism trial in atrial fibrillation: Rationale and design of the ROCKET AF study. Am Heart J 2010;159:340–347.
- Janssen Pharmaceuticals, Inc. Xarelto (rivaroxaban) package insert. Titusville, NJ; 2011.
- Husten L. Apixaban gains priority FDA review for stroke and VTE prevention in AF. Forbes (online) November 29, 2011. <https://www.forbes.com/sites/larryhusten/2011/11/29/apixaban-gains-priority-fda-review-for-stroke-and-vte-prevention-in-af/> Accessed January 5, 2012.
- Lopes RD, Alexander JH, Al-Khatib SM, et al. Apixaban for reduction in stroke and other thromboembolic events in atrial fibrillation (ARISTOTLE) trial: Design and rationale. Am Heart J 2010;159:331–339.
- Granger CB, Alexander JH, McMurray JJV, et al. Apixaban versus warfarin in patients with atrial fibrillation. N Engl J Med 2011;365:981–992.
- Eikelboom JW, O’Donnell M, Yusuf S, et al. Rationale and design of AVERROES: Apixaban versus acetylsalicylic acid to prevent stroke in atrial fibrillation patients who have failed or are unsuitable for vitamin K antagonist treatment. Am Heart J 2010;159:348–353.
- Connolly SJ, Eikelboom J, Joyner C, et al. Apixaban in patients with atrial fibrillation. N Engl J Med 2011;364:806–817.
- Carriero J, Ansell J. Apixaban, an oral direct Factor Xa inhibitor: awaiting the verdict. Expert Opin Investig Drugs 2008;17:1937–1945.
- Schirmer SH, Baumhäkel M, Neuberger HR, et al. Novel anticoagulants for stroke prevention in atrial fibrillation. J Am Coll Cardiol 2010;56:2067–2076.
- Cotton BA, McCarthy JJ, Holcomb JB. Acutely injured patients on dabigatran. N Engl J Med 2011;365:2039–2040.
- Merck and Portola Pharmaceuticals. Phase 2 study showed investigational factor Xa inhibitor, betrixaban, reduced incidence of bleeding compared to warfarin in patients with atrial fibrillation. Press release March 15, 2010. <https://portola.com/pdfs/Betrixaban_EXPLORE_ACC_032010.pdf> Accessed January 8, 2012.
- Lu G, DeGuzman MJ, Karbarz SJ, et al. Reversal of rivaroxaban-mediated anticoagulation in animal models by a recombinant antidote protein (r-Antidote, PRT064445). European Society of Cardiology 2011. Abstract 3715.
- University Hospital, Grenoble. Study in healthy volunteers of the reversion by haemostatic drugs of the anticoagulant effect of new anti-thrombotics (REVNEWANTICO). ClinicalTrials.gov. Bethesda, MD: National Library of Medicine. <https://clinicaltrials.gov/ct2/show/NCT01210755?term=NCT01210755&rank=1> Accessed January 8, 2012.











