Occlusion Perfusion Catheter (OPC): A Universal Drug-Delivery Device--Next Generation
Editor’s Note
Pradeep K. Nair, MD, FACC, FSCAI, New Technologies Section Editor
The era of drug-coated balloon (DCB) technology is in full swing. Despite the enthusiasm established by clinical trials and increased adoption among many operators who favor the “leave nothing behind” approach, we still have quite a bit to learn about the role of DCBs in peripheral artery disease (PAD) interventions. One specific question that arises is the potential negative effect of downstream washout of antiproliferative drug and the futility of clinical trials to date in demonstrating efficacy for below-the-knee disease. Additionally, cost considerations must be factored in when dealing with long lesions requiring multiple DCBs as well as any potential safety considerations that may result from drug delivery beyond the treatment zone.
As we continue to evolve in the DCB era, novel technologies are being developed that aim to address the concerns of contemporary DCBs with the goal of improving the way antiproliferative drug is delivered to improve efficacy and clinical outcomes. In this edition of New Technologies in Vascular Disease Treatment, we are privileged to have Drs Rex Teeslink and Saami Yazdani present the Occlusion Perfusion catheter (OPC), a universal drug-delivery device. This catheter allows for delivery of antiproliferative drug to a targeted treatment area, demonstrates exceptional medial layer drug uptake, and can be re-used for multiple treatment sites. Additionally, the outer occlusion balloons prevent downstream washout of anti-proliferative agent. Ongoing trials will help establish its role in PAD intervention for both above- and below-the-knee applications.
As the clinical data emerge, we will hopefully gain much more insight into optimal drug delivery. We congratulate the entire team involved with the OPC. This is a novel and innovative technology that we are certainly excited to learn more about in the months ahead.
The treatment of peripheral arterial disease (PAD) costs the United States roughly $21 billion annually and affects more than 5,000,000 people. Percutaneous transluminal angioplasty (PTA) is the most common mode of endovascular treatment for PAD and its most severe manifestation, critical limb ischemia (CLI).1 CLI, associated with extensive atherosclerotic disease of below-the-knee (BTK) vessels, is burdened by high morbidity and mortality.2-4 Treatment of these vessels presents a great challenge to endovascular interventionist due to the high incidence of long chronic total occlusions and calcified lesions; it has historically been associated with high restenosis rates and poor long-term clinical patency.5
 The recent advent of drug-coated balloons (DCBs) was designed to overcome the limitations of stents and balloon angioplasty in the treatment of PAD.6,7 Clinical DCB trials have shown promising results in the treatment of femoropopliteal disease; however, in the BTK arteries, results have been limited and long-term success has yet to be determined. DCBs were designed similar to drug-eluting stents, as antiproliferative agents are stored in the intima. Mechanistically, this concept is flawed since the deposition of drugs on the intima leads to delayed healing and incomplete endothelialization, prolonging thrombosis. Novel catheters that can deliver therapeutic agents directly to the medial wall, treat multiple lesions, and minimize any drug loss during the process offer clear advantages over DCBs.
The recent advent of drug-coated balloons (DCBs) was designed to overcome the limitations of stents and balloon angioplasty in the treatment of PAD.6,7 Clinical DCB trials have shown promising results in the treatment of femoropopliteal disease; however, in the BTK arteries, results have been limited and long-term success has yet to be determined. DCBs were designed similar to drug-eluting stents, as antiproliferative agents are stored in the intima. Mechanistically, this concept is flawed since the deposition of drugs on the intima leads to delayed healing and incomplete endothelialization, prolonging thrombosis. Novel catheters that can deliver therapeutic agents directly to the medial wall, treat multiple lesions, and minimize any drug loss during the process offer clear advantages over DCBs.
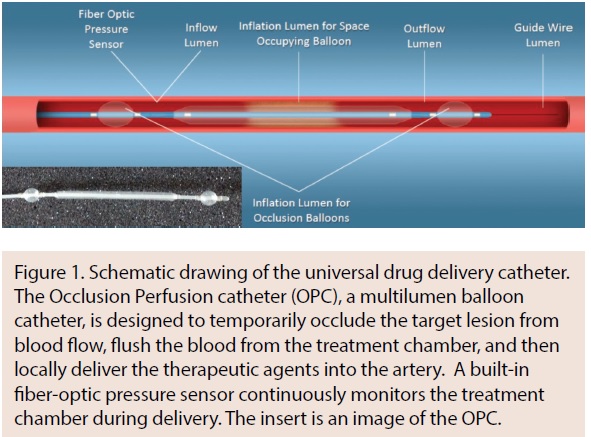
The Occlusion Perfusion catheter (OPC; Advanced Catheter Therapies) is a universal delivery catheter capable of delivering paclitaxel to the medial layer. The OPC delivers therapeutic agents by creating a treatment chamber between two occlusion balloons through which the agent is delivered. The delivery of the therapeutic agent is mechanically driven, using pressure that can be measured inside the treatment chamber (Figure 1). It was hypothesized that using this approach, paclitaxel could be delivered uniformly both circumferentially and longitudinally into the vessel wall, which has been confirmed in preclinical studies.
Technical/engineering aspects of the OPC design
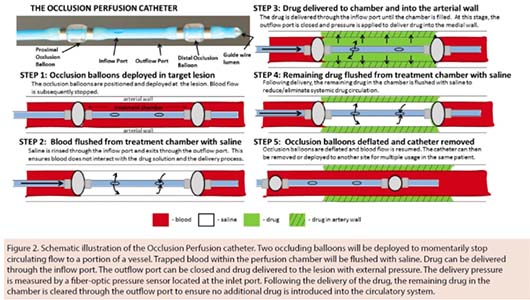
The OPC, a multilumen balloon catheter, is designed to temporarily occlude the target lesion from blood flow, flush the blood from the treatment chamber, and then locally deliver the therapeutic agents into the artery (Figure 2). A built-in pressure sensor continuously monitors the chamber during delivery. Unlike DCBs, the OPC is designed to deliver an agent to the media of the vessel wall, circumferentially and longitudinally.
The design of the OPC arrived from years of clinical practice and the realization of the limitations and potential complications of balloon angioplasty, stents, and DCBs. These limitations included the inability to treat multiple lesions with a single device, drug loss, minimal flexibility in agent delivery, etc. The solution to overcome these limitations seemed to be a universal drug-delivery device. Based on the simple idea of a universal drug delivery system, a set of requirements was developed to guide the design and ultimately provide an effective drug-delivery system to treat peripheral disease (Table 1). In 2008, Advanced Catheter Therapies was established and the OPC became a reality.
Bench-top & preclinical assessment of the OPC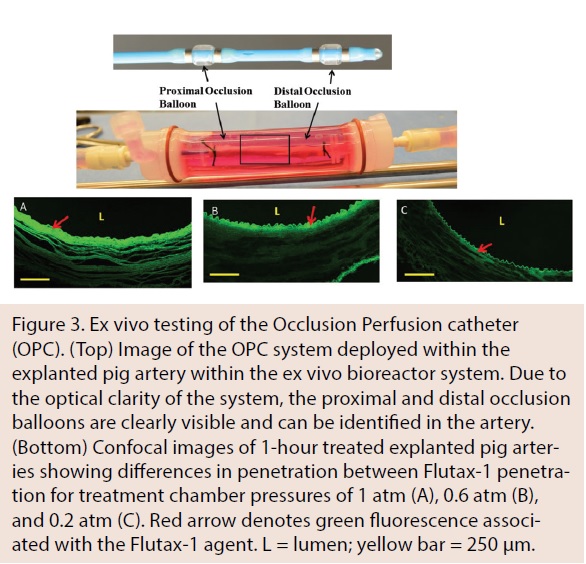
The initial feasibility and safety assessment of the OPC was performed using a set of bench-top and preclinical studies to visualize the distribution and retention of paclitaxel within the arterial wall and measure quantitatively the uptake of paclitaxel and vascular response. For these studies, paclitaxel was selected as the antiproliferative agent due to its proven clinical effectiveness.
Bench-top experiments to evaluate the OPC were conducted in a novel ex vivo system capable of rapidly evaluating arterial drug levels and visualizing drug penetration. Figure 3 shows a representative image of a porcine carotid artery being treated by a perfusion catheter within the ex vivo bioreactor system. Confocal analysis demonstrated that fluorescent paclitaxel (Flutax-1) was delivered to the artery in a uniform pattern. Additionally, ex vivo results demonstrated variable drug penetration based on treatment chamber pressure, which can be monitored and measured with the OPC.
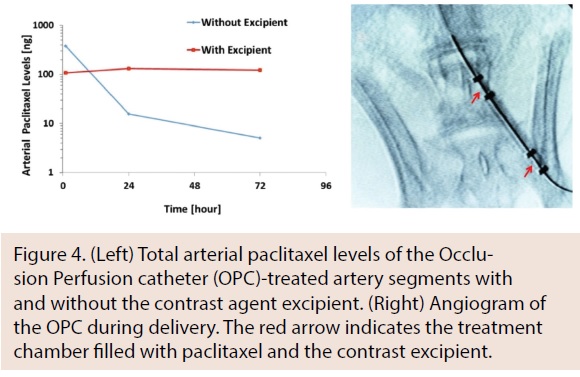 Following ex vivo studies, in vivo studies were performed using the rabbit ilio-femoral injury model to determine the impact of excipient on arterial paclitaxel retention. Arteries were treated with either paclitaxel alone or paclitaxel with an excipient using the OPC. An angiogram of the OPC within the ilio-femoral artery is shown in Figure 4. In comparing initial paclitaxel loading at 1 hour to 3 days, a significant decrease in the non-excipient group was observed (1 hr: 246.9 ± 120.3 ng vs 3 days: 2.92 ±2.91 ng; P =.01), whereas no significant decrease was observed with the excipient group (1 hr: 107.7 ± 62.1 ng vs 3 days: 40 ± 23.0 ng; P=.82). Results from the bench-top and animal models demonstrated our capability to deliver paclitaxel in a uniform manner and at therapeutic levels into the medial layer wall.
Following ex vivo studies, in vivo studies were performed using the rabbit ilio-femoral injury model to determine the impact of excipient on arterial paclitaxel retention. Arteries were treated with either paclitaxel alone or paclitaxel with an excipient using the OPC. An angiogram of the OPC within the ilio-femoral artery is shown in Figure 4. In comparing initial paclitaxel loading at 1 hour to 3 days, a significant decrease in the non-excipient group was observed (1 hr: 246.9 ± 120.3 ng vs 3 days: 2.92 ±2.91 ng; P =.01), whereas no significant decrease was observed with the excipient group (1 hr: 107.7 ± 62.1 ng vs 3 days: 40 ± 23.0 ng; P=.82). Results from the bench-top and animal models demonstrated our capability to deliver paclitaxel in a uniform manner and at therapeutic levels into the medial layer wall.
Early clinical results
 Clinical testing of the OPC was initiated in 2015. A prospective, multicenter, first-in-human registry of a novel delivery catheter delivering liquid paclitaxel was conducted in 10 patients. The primary efficacy endpoint at 6 months was freedom from clinically driven target-lesion revascularization (CD-TLR) and the primary safety endpoint at 1, 3, and 6 months was thrombosis, major amputation in the target limb, and target-limb related death.
Clinical testing of the OPC was initiated in 2015. A prospective, multicenter, first-in-human registry of a novel delivery catheter delivering liquid paclitaxel was conducted in 10 patients. The primary efficacy endpoint at 6 months was freedom from clinically driven target-lesion revascularization (CD-TLR) and the primary safety endpoint at 1, 3, and 6 months was thrombosis, major amputation in the target limb, and target-limb related death.
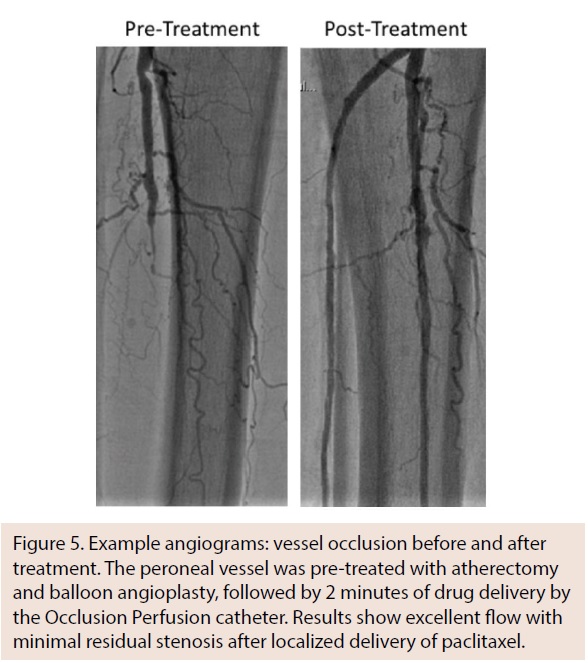
All patients tolerated the procedure well, with no reports of adverse procedural events. Twelve lesions in 10 patients were treated (mean lesion length, 83.3 ± 49.2 mm; range, 30-182 mm). At 6-month follow-up, the CD-TLR rate was 30% (3 out of 10 patients). Zero out of 10 patients demonstrated thrombosis, major amputation in the target limb, and target-limb related death at the 1-month, 3-month, and 6-month follow-up intervals. An example of a lesion angiogram before and after treatment is shown in Figure 5.
This first-in-human experience obtained in a multicenter study of real-world de novo and restenotic lesions demonstrates a favorable safety and efficacy profile at 6 months. A randomized comparison to current DCBs should be performed to further validate this approach and positive experience.
Discussion
The initial experimental and clinical studies provide the first evidence of the capability of the OPC to deliver liquid paclitaxel uniformly into the medial wall, as well as the feasibility, safety, and initial efficacy of paclitaxel administered using a universal delivery catheter for the prevention of restenosis in infrapopliteal de novo and restenotic lesions. Device success, defined as the ability to deliver paclitaxel to the interventional treatment area as intended, was 93% in the initial clinical study. The primary safety outcome demonstrated no treatment-related deaths, thrombosis, or major amputation. The CD-TLR rate was 30% in lesions with a mean length of 83.3 ± 49.2 mm. Together, these initial clinical results show the promise of such technology to treat heavy peripheral atherosclerotic disease, particularly for BTK applications.
The safety and utility profile of the OPC offers a promising option to utilize this device for the treatment of infrapopliteal lesions in CLI patients. The design of the OPC system ensures no loss of paclitaxel during tracking of the device to the lesion, as the drug is not infused at the treatment location until the treatment chamber has been established by inflation of the occlusion balloons. The absence of drug coatings and the use of liquid paclitaxel minimizes the risk of embolization, as observed in DCB studies.8 In comparison to a DCB, there is minimal barotrauma during drug delivery by the OPC, as balloon sizing is not a factor. The drug is delivered directly to the medial layer and multiple lesions can be treated with one device. This single-dose approach works well due to the potency and hydrophobicity of paclitaxel, facilitating a rapid cellular uptake and long-lasting effect on smooth muscle cell proliferation and migration.9
Future perspective
The experience obtained from a combination of bench-top and multicenter clinical studies treating de novo and restenotic lesions has demonstrated OPC safety and efficacy. The delivery of liquid paclitaxel using the OPC under controlled pressure has been shown to be technically achievable without procedural complications. The feasibility and initial efficacy of this device provide encouragement for this new technique, with the potential to be an alternative approach for peripheral occlusive disease revascularization. In particular, the ability to treat very long or multiple lesions with a single device can provide a more economical option. Although only a small cohort of clinical studies has been published, the safety profile is particularly favorable in view of recent concerns regarding adverse events with DCB for BTK applications. Longer-term follow-up and larger clinical studies will be needed and is currently ongoing to support the recent clinical findings of this technology with head-to-head comparisons between DCB and balloon angioplasty.
Acknowledgments. The authors would like to recognize Dirk Hoyns, co-founder of Advanced Catheter Therapies. Dirk was instrumental in engineering the OPC based on the idea and criterion set for developing the universal drug-delivery system.
Funding: This work was partially supported by the American Heart Association (#15SDG25880000); the National Institutes of Health (#1R15HL127596); and a research grant from Advanced Catheter Therapies.
Disclosure: The authors have completed and returned the ICMJE Form for Disclosure of Potential Conflicts of Interest. Dr Teeslink holds a patent for the Occlusion Perfusion catheter and reports personal fees from Advanced Catheter Therapies from his roles as Inventor/Co-Founder/Scientific Advisor. Dr Yazdani is on the Scientific Advisory Boards of
Advanced Catheter Therapies and Toray Industries and reports grants from Toray and Bard-Lutonix.
Manuscript submitted August 22, 2017, final version accepted August 29, 2017.
Address for correspondence: Saami K. Yazdani, PhD, Assistant Professor Mechanical Engineering Department, University of South Alabama, Mobile, AL 36688. Email: syazdani@southalabama.edu
REFERENCES
- Hirsch AT, Duval S. Effective vascular therapeutics for critical limb ischemia: a role for registry-based clinical investigation, Circ Cardiovasc Interv. 2013;6:8-11.
- Liistro F, Porto I, Angioli P, et al. Drug-eluting balloon in peripheral intervention for below-the-knee angioplasty evaluation (DEBATE-BTK): a randomized trial in diabetic patients with critical limb ischemia, Circulation. 2013;128:615-621.
- Clair D, Shah S, Weber J. Current state of diagnosis and management of critical limb ischemia. Curr Cardiol Rep. 2012;14:160-170.
- Faglia E, Clerici G, Clerissi J, et al. Long-term prognosis of diabetic patients with critical limb ischemia: a population-based cohort study, Diabetes Care. 2009;32: 822-827. Epub 2009 Feb 17.
- Adam DL, Bradbury AW. TASC II document on the management of peripheral arterial disease, Eur J Vasc Endovasc Surg. 2007;33:1-2.
- Duda SH, Poerner TC, Wiesinger B, et al. Drug-eluting stents: potential applications for peripheral arterial occlusive disease, J Vasc Interv Radiol 2003;14:291-301.
- Scheinert D, Duda S, Zeller T, et al, The LEVANT I (Lutonix paclitaxel-coated balloon for the prevention of femoropopliteal restenosis) trial for femoropopliteal revascularization: first-in-human randomized trial of low-dose drug-coated balloon versus uncoated balloon angioplasty. JACC Cardiovasc Interv. 2014;7:10-19.
- Kolodgie FD, Pacheco E, Yahagi K, Mori H, Ladich E, Virmani R. Comparison of particulate embolization after femoral artery treatment with IN.PACT Admiral versus Lutonix 035 paclitaxel-coated balloons in healthy swine. J Vasc Interv Radiol. 2016;27:1676-1685.
- Axel DI, Kunert W, Goggelmann C, et al. Paclitaxel inhibits arterial smooth muscle cell proliferation and migration in vitro and in vivo using local drug delivery. Circulation. 1997;96:636-645.
 Rex Teeslink, MD
Rex Teeslink, MD
Inventor/Co-Founder/Scientific Advisor
Advanced Catheter Therapies, Inc.
Rex Teeslink, MD, is a Vascular and Interventional Radiologist with over 40 years of experience. An honor graduate of Tulane University School of Medicine, Dr. Teeslink went on to do his Residency in Radiology at the University of Virginia in Charlottesville, where he served as Chief Resident.
Dr Teeslink began his practice as Chief of Vascular and Interventional Radiology at the Medical College of Georgia in 1968. In addition to his appointment at MCG, he was the Senior Vascular and Interventional Radiologist at the Veterans Administration Hospital, and all private hospitals in Augusta, Georgia. He was also a consultant for Eisenhower Army Medical Center. He served in these capacities for 30 years.
Dr Teeslink has been actively involved throughout his career in cutting-edge technology and continues to assist in the design, development, and implementation of many medical devices and x-ray equipment. He has been instrumental in developing many of the interventional procedures that are utilized today. Dr Teeslink is currently serving as medical director and consultant for several companies.
Dr Teeslink has always been involved in medical educational endeavors, including training medical students, residents, fellows, and postgraduate physicians. He has shared his expertise and experience at numerous medical conferences, both nationally and internationally, including being a guest speaker at the International Society of Radiology in Beijing, China in 1996. He was Co-Founder of the Southeastern Angiographic Society in 1972.
Dr Teeslink continues his medical endeavors as a consultant and is currently involved in the design and development of several new medical devices utilized in the treatment of vascular disease.
 Saami K. Yazdani, PhD
Saami K. Yazdani, PhD
Assistant Professor, Mechanical Engineering Department
University of South Alabama, Mobile, Alabama.
Dr Saami K. Yazdani is a highly proven scholar with a passion to teach and mentor. Dr Yazdani is currently an Assistant Professor within the Department of Mechanical Engineering. Dr Yazdani received his Bachelor of Science and Master’s degrees in Engineering Science and Mechanics from Virginia Tech. He received his PhD in Biomedical Engineering from Wake Forest University and performed his postdoctoral training at CVPath Institute, a non-profit cardiovascular disease organization.
For the past 15+ years, Dr Yazdani has had one passion – to find safer and more effective solutions to treat cardiovascular disease. Dr Yazdani has investigated the impacts of coronary stents on local fluid dynamics, developed tissue-engineered blood vessels, and analyzed post-mortem tissue and developed and tested stents and drug-coated balloons in preclinical models. At the University of South Alabama, Dr Yazdani has continued finding novel solutions to prevent and treat cardiovascular disease. His team employs a combination of traditional engineering approaches (solid mechanics and fluid mechanics), biological techniques (tissue engineering and physiology), and drug delivery (excipients, pharmacokinetics) to develop models to better understand the pathology of cardiovascular disease, characterize the failure of cardiovascular devices, and develop new technology. His team consists of strong collaboration with engineers, biologists, clinicians, and industry.













