ADVERTISEMENT
Ablation of Atrial Fibrillation with a Coronary Sinus Trigger
In recent years, catheter ablation has become a preferred strategy for drug-refractory paroxysmal atrial fibrillation and for select patients with persistent atrial fibrillation.1 Though the field has rapidly advanced, the procedure is still limited by a relatively high recurrence rate, particularly when compared to other ablations such as that for supraventricular tachycardia. A high rate of recurrence is particularly seen in the ablation of persistent atrial fibrillation. The cornerstone of atrial fibrillation ablation is the electrical isolation of all the pulmonary veins.1 However, in approximately 20% of patients (perhaps as high as 35% in persistant AF), non-pulmonary vein triggers can be identified.2 This may, at least in part, explain the relatively high recurrence rate of arrhythmias despite complete pulmonary vein isolation at the time of the procedure. The following case report describes ablation of recurrent persistent atrial fibrillation in which a coronary sinus trigger was thought to be playing a role in the patient’s arrhythmia.
Case Description
 A 64-year-old male presented for a second ablation for persistent atrial fibrillation. He had no structural heart disease, no hypertension, and moderate left atrial enlargement with a left atrial diameter of 4.7 cm. He had initially failed dofetilide followed by ablation a year and a half prior. At that time, ablation consisted of radiofrequency pulmonary vein isolation (PVI) using an open irrigated tip catheter (ThermoCool, Biosense Webster, Inc., a Johnson & Johnson company, Diamond Bar, CA) and a double transseptal puncture, and
A 64-year-old male presented for a second ablation for persistent atrial fibrillation. He had no structural heart disease, no hypertension, and moderate left atrial enlargement with a left atrial diameter of 4.7 cm. He had initially failed dofetilide followed by ablation a year and a half prior. At that time, ablation consisted of radiofrequency pulmonary vein isolation (PVI) using an open irrigated tip catheter (ThermoCool, Biosense Webster, Inc., a Johnson & Johnson company, Diamond Bar, CA) and a double transseptal puncture, and 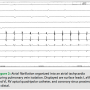 was guided by a circular mapping catheter. Ablation of complex fractionated atrial electrograms (CFAE) and a mitral isthmus line (from the anterior mitral valve annulus, along the medial aspect of the left atrial appendage to the left superior pulmonary vein), was also done. This procedure was complicated by a pericardial effusion requiring percutaneous drainage. The patient recovered from the ablation, but experienced recurrent atrial fibrillation. He was initially treated with sotalol and maintained sinus rhythm for nearly a year, but had subsequent recurrence while on sotalol. He then decided to proceed with redo ablation.
was guided by a circular mapping catheter. Ablation of complex fractionated atrial electrograms (CFAE) and a mitral isthmus line (from the anterior mitral valve annulus, along the medial aspect of the left atrial appendage to the left superior pulmonary vein), was also done. This procedure was complicated by a pericardial effusion requiring percutaneous drainage. The patient recovered from the ablation, but experienced recurrent atrial fibrillation. He was initially treated with sotalol and maintained sinus rhythm for nearly a year, but had subsequent recurrence while on sotalol. He then decided to proceed with redo ablation.
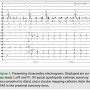
 During the second ablation, the patient presented to the electrophysiology laboratory in atrial fibrillation. After placement of the coronary sinus (CS) decapolar catheter, he was found to have CFAE in the proximal coronary sinus (Figure 1). Single transseptal access was obtained with the assistance of intracardiac echocardiography. A three-dimensional map was made and fused to a CT scan acquired the day prior to the ablation. Pulmonary vein isolation (PVI) was done using a 23 mm Arctic Front ablation catheter (Medtronic, Inc.,
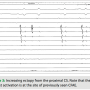
During the second ablation, the patient presented to the electrophysiology laboratory in atrial fibrillation. After placement of the coronary sinus (CS) decapolar catheter, he was found to have CFAE in the proximal coronary sinus (Figure 1). Single transseptal access was obtained with the assistance of intracardiac echocardiography. A three-dimensional map was made and fused to a CT scan acquired the day prior to the ablation. Pulmonary vein isolation (PVI) was done using a 23 mm Arctic Front ablation catheter (Medtronic, Inc., 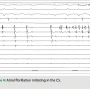 Minneapolis, MN) and a 20 mm Achieve circular mapping catheter (Medtronic, Inc.) for the left-sided veins, and a 28 mm Arctic Front with a 20 mm Achieve catheter for the right-sided veins. During ablation of the right-sided pulmonary veins, the patient’s AF organized into an atrial tachycardia (AT, Figure 2). The AT terminated with ablation of the left inferior vein. After PVI, a 20-minute waiting period was observed, after which entrance and exit block was documented in all four veins. Isoproterenol was then initiated, and at peak dose complex ectopy was noted with the earliest activation in the proximal CS (Figure 3). This then degenerated into atrial fibrillation (Figure 4), suggesting a CS trigger. Of note, the area of earliest activation correlated to an area of CFAE seen at baseline. Ablation was then done in the coronary sinus using irrigated RF at a power setting of 15–20 watts (Figure 5) until there was a substantial reduction in CS electrogram amplitude. No ablation was attempted on the endocardial left atrial aspect of the CS. Ablation in the CS failed to terminate AF, and the patient was cardioverted. However, after restoration of sinus rhythm, isoproterenol was re-initiated and titrated to the previous maximum dose, and neither CS ectopy nor AF was inducible any longer. The patient was seen one month after the ablation and has maintained sinus rhythm.
Minneapolis, MN) and a 20 mm Achieve circular mapping catheter (Medtronic, Inc.) for the left-sided veins, and a 28 mm Arctic Front with a 20 mm Achieve catheter for the right-sided veins. During ablation of the right-sided pulmonary veins, the patient’s AF organized into an atrial tachycardia (AT, Figure 2). The AT terminated with ablation of the left inferior vein. After PVI, a 20-minute waiting period was observed, after which entrance and exit block was documented in all four veins. Isoproterenol was then initiated, and at peak dose complex ectopy was noted with the earliest activation in the proximal CS (Figure 3). This then degenerated into atrial fibrillation (Figure 4), suggesting a CS trigger. Of note, the area of earliest activation correlated to an area of CFAE seen at baseline. Ablation was then done in the coronary sinus using irrigated RF at a power setting of 15–20 watts (Figure 5) until there was a substantial reduction in CS electrogram amplitude. No ablation was attempted on the endocardial left atrial aspect of the CS. Ablation in the CS failed to terminate AF, and the patient was cardioverted. However, after restoration of sinus rhythm, isoproterenol was re-initiated and titrated to the previous maximum dose, and neither CS ectopy nor AF was inducible any longer. The patient was seen one month after the ablation and has maintained sinus rhythm.
 Since AF was initiated by CS ectopy, and ablation at this site rendered AF uninducible, this suggests but does not confirm a CS trigger for this patient’s atrial fibrillation. Though AF did not terminate with ablation here, neither AF nor CS ectopy was inducible at the conclusion of the study, despite an identical isoproterenol infusion dose that previously resulted in CS ectopy and AF. Though the majority of non-PV triggers for AF are thought to be from the superior vena cava, the left atrial posterior free wall, crista terminalis, coronary sinus, ligament of Marshall, and interatrial septum have also been implicated.2,3 Haïssaguerre and colleagues demonstrated that a combination of epicardial and endocardial CS ablation can terminate CS persisting after PVI.4 Though AF did not terminate with epicardial CS ablation in our patient, no endocardial ablation was attempted.
Since AF was initiated by CS ectopy, and ablation at this site rendered AF uninducible, this suggests but does not confirm a CS trigger for this patient’s atrial fibrillation. Though AF did not terminate with ablation here, neither AF nor CS ectopy was inducible at the conclusion of the study, despite an identical isoproterenol infusion dose that previously resulted in CS ectopy and AF. Though the majority of non-PV triggers for AF are thought to be from the superior vena cava, the left atrial posterior free wall, crista terminalis, coronary sinus, ligament of Marshall, and interatrial septum have also been implicated.2,3 Haïssaguerre and colleagues demonstrated that a combination of epicardial and endocardial CS ablation can terminate CS persisting after PVI.4 Though AF did not terminate with epicardial CS ablation in our patient, no endocardial ablation was attempted.
Discussion
This case highlights the need to search for non-pulmonary vein triggers, particularity in redo ablations and in ablation of persistent atrial fibrillation. Because the vast majority of literature regarding the ablation of AF addresses the effectiveness and need for complete PVI,1 and the data for non-pulmonary vein triggers and the effect of their elimination of ablation success is limited, I do not routinely perform isoproterenol infusions after a first-time ablation for paroxysmal atrial fibrillation. However, I do routinely give isoproterenol during redo procedures. In this case, the patient also had reconnection of the pulmonary veins, and whether or not the AF seen originating from the CS was a “clinical” rhythm, I felt there was enough “circumstantial” evidence to justify ablation there. It is also interesting this site corresponded to CFAE seen at baseline, as others have identified a line between CFAE and non-PV AF triggers.5
Though ablation for the treatment of atrial fibrillation has progressed a great deal since pulmonary vein isolation was first described, the high recurrence rates and lack of consensus as to how to ablate persistent atrial fibrillation highlights how limited our understanding is of this very common arrhythmia. “Learning by burning,” whether with regard to CFAE or non-pulmonary vein triggers, has advanced our understanding and increased our effectiveness, but clearly we still have much to learn.
References
- Fuster V, Rydén LE, Cannom DS, et al. ACC/AHA/ESC 2006 Guidelines for the Management of Patients with Atrial Fibrillation: A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the European Society of Cardiology Committee for Practice Guidelines (Writing Committee to Revise the 2001 Guidelines for the Management of Patients With Atrial Fibrillation). Circulation 2006;114:e257–e354.
- Chen SA, Tai CT. Catheter ablation of atrial fibrillation originating from the non-pulmonary vein foci. J Cardiovasc Elecrophysiol 2006;16:229–232.
- Arruda M, Mlcochova H, Prasad SK, et al. Electrical isolation of the superior vena cava: An adjunctive strategy to pulmonary vein antrum isolation improving the outcome of AF ablation. J Cardiovasc Electrophysiol 2007;12:1261–1266.
- Haïssaguerre M, Hocini M, Takahashi Y, et al. Impact of catheter ablation of the coronary sinus on paroxysmal or persistent atrial fibrillation. J Cardiovasc Electrophysiol 2007;4:378–386.
- Elayi CS, Di Biase L, Bai R, et al. Identifying the relationship between the non-PV triggers and the critical CFAE sites post-PVAI to curtail the extent of atrial ablation in longstanding persistent AF. J Cardiovasc Electrophysiol 2011:1199–1205.











