ADVERTISEMENT
Can a Stroke Card System Reduce Door-to-Needle Times?
At 15:03 on an otherwise-normal afternoon in 2014, an 85-year-old male developed a sudden onset of right-sided weakness. Upon witnessing his symptoms, a family member called emergency medical services at 15:08. At 15:13 EMS arrived.
The crew’s initial exam demonstrated an alert and oriented 100-kg geriatric male with marked weakness of his right arm and leg. His past medical history included coronary artery disease, chronic obstructive pulmonary disease, deep vein thrombosis status post-IVC filter, hypertension, and chronic atrial fibrillation. As scene time progressed, the patient became progressively aphasic and altered, with a final Cincinnati Stroke Scale score of 2 for arm drift and slurred speech.
Per protocol, a prehospital 12-lead ECG was taken at 15:23, showing atrial fibrillation at a rate of 60 bpm, and a peripheral IV was placed at 15:26. While preparing to transport the patient to a comprehensive stroke center, EMS gave a stroke card to one of the patient’s family members. This card instructed the family member to call a phone number that would connect them with an on-call neurology stroke team resident.
While the patient was still with EMS, the family called the resident, who acquired the patient’s time last known normal, a brief clinical history, pertinent past medical history (noting the patient was not on anticoagulation), and consent from the family to use tissue plasminogen activator (tPA) if clinically indicated. At 15:27 EMS transported the patient to the hospital, notified the receiving facility, and triggered a stroke page.
At the Hospital
EMS arrived at the hospital at 15:37. At 15:39 the patient was transferred directly to the ED’s CT scanner for imaging studies while being evaluated by emergency medicine and neurology physicians. Initial assessment demonstrated a nonverbal patient who could not follow commands. He was hemiparetic on his right side, with right-sided facial droop and left gaze preference.
A noncontrast head CT was obtained at 15:42, marking a door-to-CT time of five minutes. A wet read at the scanner by radiology excluded intracranial hemorrhage but showed a critical finding of density in the left M1 segment concerning for acute thrombus. The patient’s initial National Institutes of Health Stroke Scale (NIHSS) score was noted to be 25 by neurology for dense hemiparesis and aphasia. Subsequent point-of-care blood glucose level testing excluded hypoglycemia (BGL=100 mg/dL).
Given the prearrival information provided by the witness, the clinical exam, and the imaging, the care team decided to give tPA. They administered the initial bolus at 16:05, marking a door-to-needle time of 28 minutes. Neurology planned a CT angiogram of the man’s head and neck to assess for possible mechanical thrombectomy. Although thrombectomy is consistent with the standard of care today, at the time of this case in 2014, it was in its infancy. But over the two hours following tPA administration, serial neurologic exams revealed progressively improving mental status, motor ability, and speech.
At 17:57 assessment by neurology revealed an alert and oriented patient who could speak in full sentences with clear and coherent speech. The patient recalled all preceding events and explained that in the scanner prior to tPA administration, he attempted to answer questions and follow commands but could not. Examination also revealed complete resolution of his right-sided hemiparesis. The formal NIHSS reassessment revealed a score of 0. Neurology cancelled the CT angiogram, and at 19:13 the patient was admitted to the neurosurgical ICU. Two days later he was discharged from the hospital with no residual neurologic deficits or complications.
Discussion
Despite advances over the last few decades, acute ischemic stroke remains a major cause of morbidity and mortality, with 795,000 strokes and 140,000 stroke deaths occurring annually in the United States.1 Stroke accounts for 14% of total health expenditures worldwide, and associated care costs an estimated $316 billion annually in the U.S. in direct costs and lost productivity.1
Tissue plasminogen activator has been a cornerstone of definitive stroke care and shown to improve neurologic outcomes in acute ischemic stroke.2,3 tPA is administered as a thrombolytic in stroke care to patients presenting within three hours of symptom onset,3 and recent American Heart Association guidelines extend that recommendation in select patients up to 4.5 hours.4 Notably, the efficacy of tPA scales significantly with time to administration,5 and guidelines recommend tPA be administered as soon as possible in eligible patients.4
At the turn of this century, the involvement of EMS personnel in the recognition and rapid transport of suspected stroke patients was limited. Public health research showed stroke patients were presenting as ineligible for tPA administration largely due to delays in arrival to definitive care that put them outside tPA’s three-hour window.6 However, initial prehospital public health efforts were largely targeted to improving layperson recognition of stroke.7 A study of paramedics’ knowledge of stroke care in 1999 demonstrated that prehospital providers understood stroke injured the brain but were largely unaware of the use of tPA as a treatment and the short window of opportunity for its administration.8
Since then education efforts and guidelines targeted to EMS have led to improvements in stroke recognition and EMS response times, with emphasis on reducing scene times and streamlining communication with receiving institutions.9–11 Importantly, the Target: Stroke initiative from the AHA places strong emphasis on advance hospital notification.12
In addition to faster recognition and transport of potential stroke patients, advance hospital notification by EMS personnel has led to the streamlining of tPA therapy at stroke care centers. Studies examining the effects of advance notification on tPA eligibility and administration time have found it decreases door-to-CT time and door-to-needle time (DNT) and increases the proportion of stroke patients eligible for tPA.13,14 Interestingly, one study showed EMS stroke alerts may have better diagnostic performance than stroke alerts by hospital personnel,15 underscoring the importance of participation by prehospital personnel in identifying and treating stroke patients.
Once stroke patients arrive at definitive care, care and assessment must be efficient and streamlined to reduce DNT and increase the likelihood the patient will benefit from early tPA. A group in Finland reported progressively streamlining their stroke alert protocols to achieve an average door-to-needle time of 20 minutes.16 The authors cited the education of dispatchers and EMS personnel and advance notification directly to stroke physicians as prehospital variables that helped improve treatment times. Specifically, EMS personnel called the stroke physician directly via a programmed cell phone, then gave the phone to a family member on scene to allow the physician to obtain a preliminary history. Additionally, this group introduced protocols to speed initial patient assessment (e.g., preordering labs and point-of-care INR), acquisition of imaging (e.g., installing CT scanners in the ED), and administration of tPA (e.g., premixing tPA and administration while on the CT table). The result of Helsinki University Central Hospital’s 12 optimization steps is one of the lowest DNTs currently published. Additionally, its stroke model has been replicated in another locale with a similar dramatic improvement in DNT.17
While our report focuses on tPA therapy for stroke as the standard of care in 2014, the modern standard also includes endovascular intervention in patients with large vessel occlusions. Importantly, the beneficial effect of reduced time to intervention in acute ischemic stroke also applies when endovascular therapy is the intervention of choice.18
Stroke Card System
At our center in 2012, we utilized Toyota’s lean manufacturing principles to assess and optimize our in-hospital stroke care workflow by removing wasteful and inefficient steps in our care procedures.19 The most time-saving change was having EMS transport the patient directly to the CT scanner instead of placing them in a patient care room before transferring to CT.19 Other improvements included point-of-care INR for patients on anticoagulants, parallel completion of the focused history and NIHSS, and early preparation of tPA by a stroke team pharmacist.19 This streamlining of our center’s stroke care workflow resulted in a significant reduction in our median DNT to 39 minutes.19
Despite this progress in both prehospital and in-hospital stroke care, obtaining the last known normal (LKN) time and pertinent medical history from a witness to the patient’s stroke remains a rate-limiting step of determining tPA administration eligibility.6,19 To ensure validity of LKN and significant medical history, many stroke systems require a direct conversation between the treating physician and the witness for verification. Secondhand communication is deemed insufficient due to the risk of an incorrect LKN, forgotten contraindications to tPA therapy from the medical history, or the risk of exposing patient health information over unsecured networks.
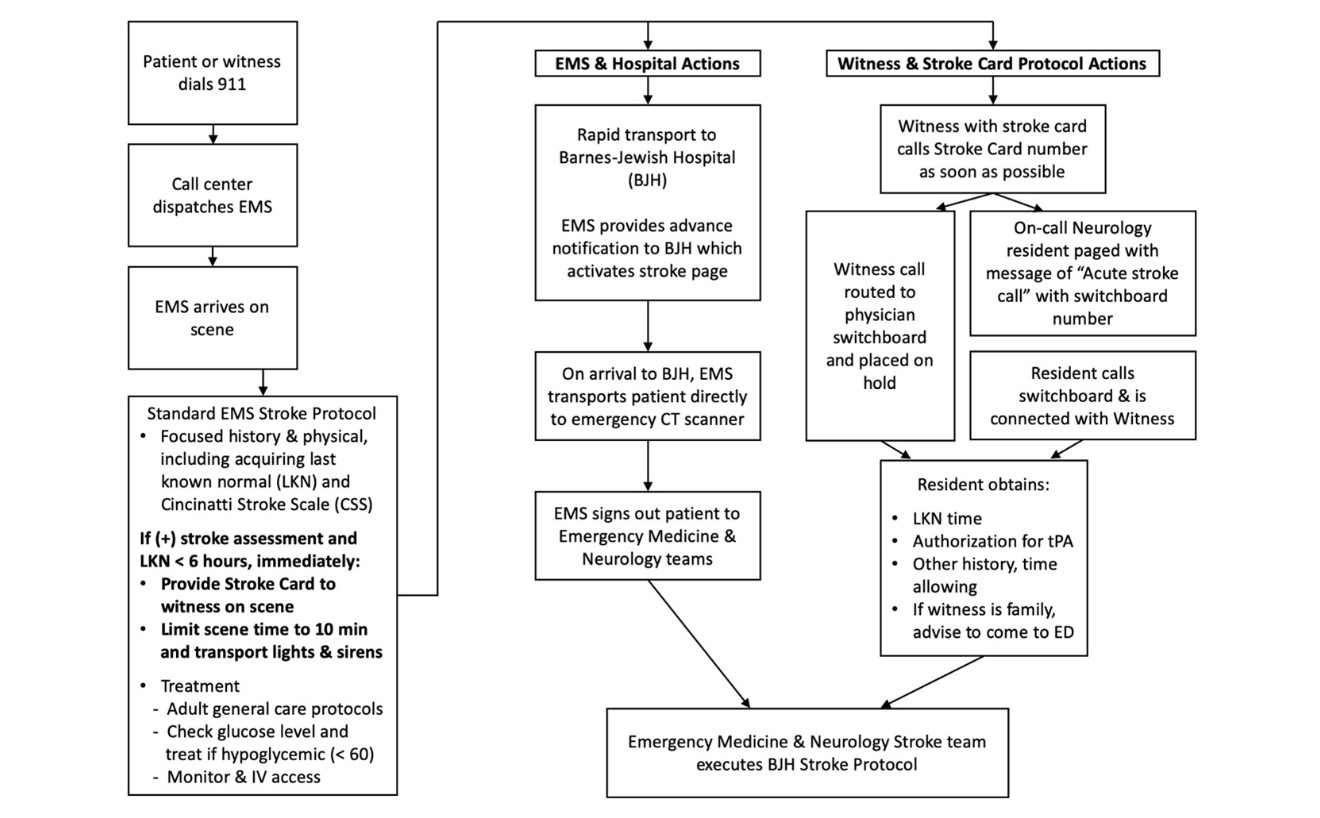
To facilitate rapid acquisition of the LKN and brief medical history from a witness, we trialed a novel cost-effective stroke card system. In this system prehospital providers carried stroke cards (Figures 1a, 1b) that included a phone number to call to reach the on-call neurology resident and a short description of the information the witness was asked to provide. Once a patient was recognized as having strokelike symptoms, the prehospital provider would provide a stroke card to the witness and proceed with the rapid assessment and transportation of the patient (Figure 2 represents the complete workflow). Our hypothesis was that determining the LKN and checking for contraindications to tPA prior to or soon after patient arrival to the hospital would further streamline the determination of tPA eligibility and facilitate shorter DNTs.
The opening patient experienced a DNT of 28 minutes, resulting in complete resolution of right-sided hemiparesis and severe aphasia with no residual deficits after probable occlusion of the left M1 segment. Several factors led to this patient’s complete recovery: The patient had an acute onset of symptoms in the presence of family, who immediately noticed his right-sided weakness and called EMS within five minutes. With the patient’s clear right-sided weakness and progressive aphasia on scene, the prehospital providers correctly determined stroke as their working diagnosis, prepared the patient for transport, and gave a family member a stroke card. At some point during EMS care, the family member successfully contacted the on-call neurology resident and provided the LKN and focused history, eliminating a critical rate-limiting step to tPA administration. Once at Barnes-Jewish Hospital, within five minutes of door time, the patient received a noncontrast head CT. Over the subsequent 20 minutes, further assessment and point-of-care labs confirmed his tPA eligibility, and at 16:05, 28 minutes after door time, the patient received his bolus loading dose of tPA. The rapid acquisition of pertinent history, aided by a cost-effective stroke card system, contributed to this shortened DNT and likely to his notable and rapid neurologic recovery.
Challenges and Concerns
Despite the marked improvement in DNT seen in this patient case, our stroke card system presented numerous challenges that ultimately resulted in its cessation. A summary of those is presented in Table 1. Acquisition of the LKN from witnesses with low health literacy proved difficult when they couldn’t understand the stroke card instructions or questions being asked by the on-call neurology resident. Indeed, low health literacy is described in the literature as an impediment to determining LKN in stroke patients.20 Other challenges included problems connecting the witness and the neurology resident, witnesses not receiving stroke cards, and witnesses opting not to utilize stroke cards.
Another important consideration is that city EMS transport times to our center are generally short. With short transport times, the effectiveness of the stroke card in reducing DNT is limited. We observed many cases in which a stroke card call was not useful because by the time the call occurred, either the patient or the patient’s family had already arrived and relayed the required information directly to the care team. However, in systems that have long transport times, particularly rural EMS, the stroke card may be more advantageous, as it enables advance hospital notification well before patient arrival. Additionally, the cost of producing a stroke card can be a practical alternative to expensive mobile stroke units or telemedicine technologies. The system in this case resulted in a markedly low DNT, and because the efficacy of tPA scales with how soon it is administered after symptom onset,5 lowering DNT could improve patient outcomes in a cost-effective manner.
However, several improvements are necessary prior to wider implementation. First, population health literacy must be reviewed, and instructions and interview questions streamlined to accommodate the health literacy of likely witnesses. Second, some witnesses given stroke cards didn’t have cellular phones to utilize, and a system to facilitate phone calls for such witnesses may improve participation rates. Third, the calling system should be optimized so witnesses aren’t placed on hold. Lastly, recurrent education should be implemented for EMS, neurology residents taking calls, and receiving ED physicians. With the implementation of this cost-effective stroke card system, healthcare providers can continue to streamline stroke care, reduce DNTs, and optimize tPA administration for stroke patients.
References
1. Benjamin EJ, Muntner P, Alonso A, et al. Heart Disease and Stroke Statistics—2019 Update: A Report From the American Heart Association. Circulation, 2019; 139: e56–e528.
2. Hacke W, Kaste M, Fieschi C, et al. Intravenous Thrombolysis With Recombinant Tissue Plasminogen Activator for Acute Hemispheric Stroke: The European Cooperative Acute Stroke Study. JAMA, 1995; 274(13): 1,017–25.
3. National Institute of Neurological Disorders and Stroke rt-PA Stroke Study Group. Tissue plasminogen activator for acute ischemic stroke. NEJM, 1994; 333(24): 1,581–7.
4. Powers WJ, Rabinstein AA, Ackerson T, et al. Guidelines for the Early Management of Patients With Acute Ischemic Stroke: 2019 Update to the 2018 Guidelines for the Early Management of Acute Ischemic Stroke: A Guideline for Healthcare Professionals From the American Heart Association/American Stroke. Stroke, 2019; 50: e344–e418.
5. The Atlantis, ECASS, and NINDS rt-PA Study Group. Association of outcome with early stroke treatment: pooled analysis of ATLANTIS, ECASS, and NINDS rt-PA stroke trials. Lancet, 2004; 363: 768–74.
6. Barber PA, Zhang J, Demchuk AM, Hill MD, Buchan AM. Why are stroke patients excluded from TPA therapy? An analysis of patient eligibility. Neurology, 2001; 56: 1,015–20.
7. Pepe PE, Zachariah BS, Sayre MR, Floccare D. Ensuring the chain of recovery for stroke in your community. Prehosp Emerg Care, 1998; 2(2): 89–95.
8. Crocco TJ, Kothari RU, Sayre MR, Liu T. A nationwide prehospital stroke survey. Prehosp Emerg Care, 1999; 3(3): 201–6.
9. Schwartz J, Dreyer RP, Murugiah K, Ranasinghe I. Contemporary Prehospital Emergency Medical Services Response Times for Suspected Stroke in the United States. Prehosp Emerg Care, 2016; 20(5): 560–5.
10. Oostema JA, Chassee T, Reeves M. Emergency Dispatcher Stroke Recognition: Associations with Downstream Care. Prehosp Emerg Care, 2018; 22(4): 466–71.
11. Adeoye O, Nyström KV, Yavagal DR, et al. Recommendations for the Establishment of Stroke Systems of Care: A 2019 Update: A Policy Statement from the American Stroke Association. Stroke, 2019; 50: e1–e24.
12. Xian Y, Xu H, Lytle B, et al. Use of Strategies to Improve Door-to-Needle Times with Tissue-Type Plasminogen Activator in Acute Ischemic Stroke in Clinical Practice: Findings from Target: Stroke. Circ Cardiovasc Qual Outcomes, 2017; 10: 1–8.
13. Abdullah AR, Smith EE, Biddinger PD, Kalenderian D, Schwamm LH. Advance hospital notification by EMS in acute stroke is associated with shorter door-to-computed tomography time and increased likelihood of administration of tissue-plasminogen activator. Prehosp Emerg Care, 2008; 12: 426–31.
14. Kim SK, Lee SY, Bae HJ, et al. Pre-hospital notification reduced the door-to-needle time for iv t-PA in acute ischaemic stroke. Eur J Neurol, 2009; 16: 1,331–5.
15. Medoro I, Cone DC. An Analysis of EMS and ED Detection of Stroke. Prehosp Emerg Care, 2017; 21(4): 476–80.
16. Meretoja A, Strbian D, Mustanoja S, et al. Reducing in-hospital delay to 20 minutes in stroke thrombolysis. Neurology, 2012; 79: 306–13.
17. Wu TY, Coleman E, Wright SL, et al. Helsinki stroke model is transferrable with “real-world” resources and reduced stroke thrombolysis delay to 34 min in Christchurch. Front Neurol, 2018; 9(290): 1–8.
18. Meretoja A, Keshtkaran M, Tatlisumak T, Donnan GA, Churilov L. Endovascular therapy for ischemic stroke. Neurology, 2015; 88: 2,123–7.
19. Ford AL, Williams JA, Spencer M, et al. Reducing door-to-needle times using Toyota’s lean manufacturing principles and value stream analysis. Stroke, 2012; 43: 3,395–8.
20. Carpenter CR, Kaphingst KA, Goodman MS, et al. Feasibility and diagnostic accuracy of brief health literacy and numeracy screening instruments in an urban emergency department. Acad Emerg Med, 2014; 21(2): 137–46.
Table 1: Challenges of the Stroke Card Program
Witness-Related
- Witness could not understand stroke card instructions
- Witness could not understand questions posed by neurology resident
- Witness did not have a phone to call from
- Witness opted to drive to the hospital instead of utilizing the stroke card
Process-Related
- Neurology resident wasn’t expecting witness call because stroke alert hadn’t occurred
- Hospital call system placed witness on hold and witness hung up
- Prehospital provider forgot to provide stroke card
- Prehospital provider did not have impression of stroke and thus did not provide stroke card
- New prehospital provider did not know the stroke card system was active
- Short average transport times limit effectiveness of stroke card in reducing DNT
Sidebar: Events in Patient Care From First Contact to Symptom Resolution
Time—Event or Intervention
15:03—Patient experiences witnessed sudden onset of right-sided weakness
15:08—EMS dispatched
15:13—EMS on scene
NR—EMS gives stroke card to witness
15:23—EMS obtains 12-lead ECG showing atrial fibrillation
15:26—EMS places 20G peripheral IV
15:27—EMS en route with patient to Barnes-Jewish Hospital; receiving hospital notified; tPA stroke pager activated
NR—Family member calls stroke team resident, who obtains time last known normal
15:37—EMS arrives at Barnes-Jewish Hospital (door time)
15:39—Assessment by emergency medicine and neurology begins in CT scanner
NR—NIHSS noted to be 25
15:42—Noncontrast head CT obtained, excluding intracranial hemorrhage with finding of density in left M1 segment “concerning for thrombus”
15:48—Point-of-care INR result completed (INR = 1.0)
15:51—Point-of-care blood glucose result completed (BGL = 110 mg/dL)
16:04—ED physician obtains 12-lead ECG showing atrial fibrillation
16:05—tPA administered (needle time)
16:10—Neurology plan to admit to neurosurgical ICU and obtain CT angiogram of head and neck to evaluate for possible mechanical thrombectomy
17:57—Post-tPA neuro exam shows fully alert and oriented patient able to speak in full sentences with clear speech, memory completely intact, and resolution of right-sided weakness
18:00—Neurology cancels CT angiogram, plans to admit to neurosurgical ICU for post-tPA monitoring
18:10—Repeat NIHSS noted to be 0
19:13—Patient admitted to neurosurgical ICU at neurologic baseline
NR = exact time not recorded
Ryan D. Pappal, MD, NRP, is an emergency medicine resident at Washington University School of Medicine in St. Louis.
Hawnwan Philip Moy, MD, is an assistant medical director of the St. Louis City Fire Department and emergency medicine clinical instructor and core faculty in the EMS Section of the Division of Emergency Medicine at Washington University in St. Louis.
Laura Heitsch, MD, is assistant professor of emergency medicine at Washington University School of Medicine in St. Louis.
Peter Panagos, MD, is professor of emergency medicine at Washington University School of Medicine in St. Louis.
David Curfman, MD, is a vascular neurology specialist at Neurology Associates, Missouri Baptist Medical Center.