Optimizing Revascularization Outcomes and Limb Preservation in Critical Limb Ischemia
Today there are over 12 million patients with peripheral artery disease (PAD) in the United States.1,2 The most severe form of PAD is critical limb ischemia (CLI). Presentations of CLI that require hospitalization lead to great morbidity and mortality, with 29% undergoing major amputation or death at 12 months.3 Further, greater than 50% of patients with CLI who undergo major lower-extremity amputation have no revascularization performed in the year before amputation, adding to the mortality associated with this disease.3,4 Revascularization not only saves the affected limb, but is associated with reduced readmissions and costs.3 Revascularization, however, involves the need for advanced endovascular therapy skills and tools to improve outcomes. One such tool is the HawkOne Directional Atherectomy system (Medtronic). This device has the versatility to treat lesion morphologies above the knee, below the knee, and even into the pedal arch. Additionally, the low profile 6 French (Fr) options allow for treatment using alternative access sites. Directional atherectomy has also proven to be effective in preventing major limb amputation in CLI patients. In the DEFINITIVE LE trial, enrolling 800 subjects, the freedom from major unplanned amputation of the target limb at 12 months in CLI subjects was 95%.5 In this article, three unique clinical scenarios are presented in which endovascular therapy prevented major amputations and improved patient outcomes.
Hear from Dr. Rao, below:
Case 1
A white female in her early 40s presented with progressive right foot gangrene for six weeks (Figure 1, left). Significant comorbidities included type 2 diabetes, smoking, hypertension, hyperlipidemia, and dilated cardiomyopathy, with an ejection fraction of 40%. Arterial duplex presented no evidence of flow-limiting right femoropopliteal disease. Tardus-parvus waveforms were noted in all three tibial vessels. Toe pressures were not detected. The patient subsequently underwent a trans-metatarsal amputation since there was significant gangrenous tissue.

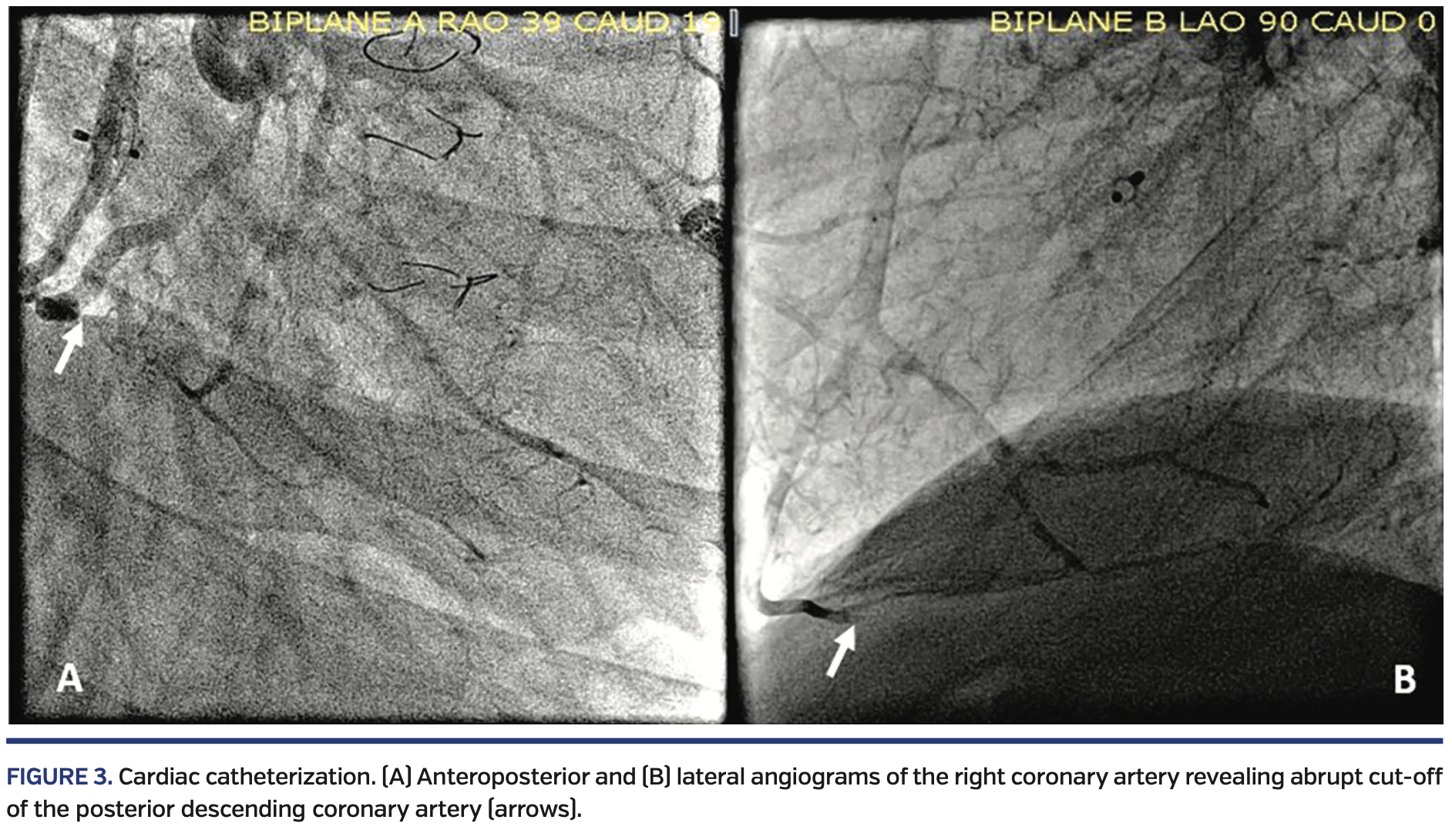
Right lower extremity angiography via antegrade right common femoral artery access showed severe, flow-limiting, 3-vessel tibial disease on the right side, with occluded distal anterior tibial and posterior tibial arteries (Figure 2).

The occlusions in the arteries were crossed with a Fielder FC wire (Asahi-Intecc) and directional atherectomy with a HawkOne S Directional atherectomy system (Medtronic) was performed through the occluded posterior tibial, through the pedal arch, and into the dorsalis pedis artery (Figure 3).

Due to residual eccentric disease in the anterior tibial and dorsalis pedis arteries, atherectomy was performed with the same device from the anterior tibial into the posterior tibial via the pedal arch, followed by balloon angioplasty with a 2.5 x 220 mm Coyote balloon (Boston Scientific), resulting in 0% residual stenosis and restoration of normal flow in the pedal arch (Figure 4).

The patient was subsequently discharged home with a healthy surgical site (Figure 1, right) and was able to ambulate with a cane.
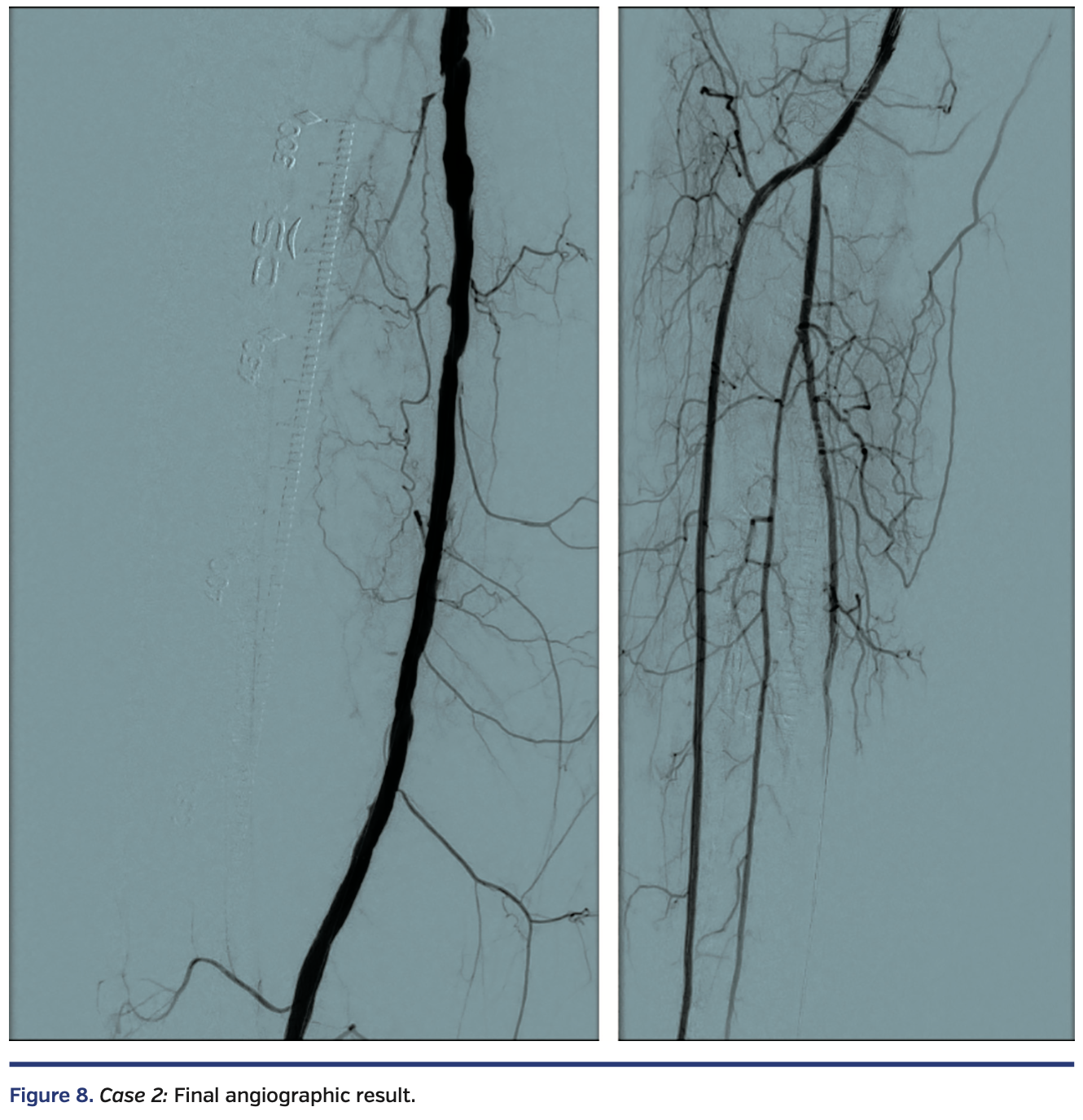
Case 2
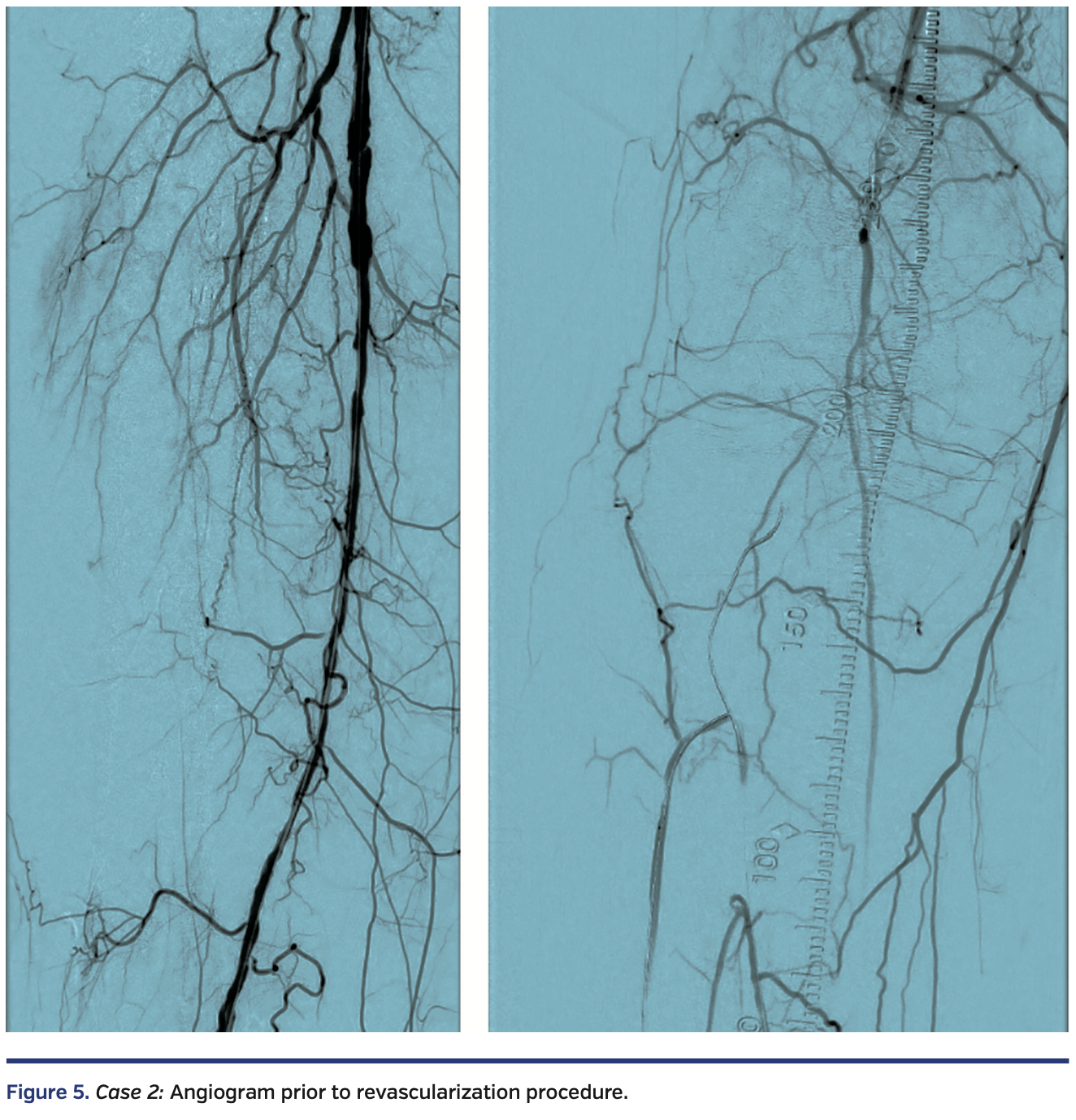
A functional white female in her late eighties with history of type 2 diabetes, hypertension, and hyperlipidemia presented with progressive right hallux discoloration and early pre-gangrenous changes over a 2-month period. Arterial duplex suggested a right popliteal artery (P3 segment) occlusion, distal posterior tibial artery occlusion, and patent anterior tibial and peroneal arteries. Diagnostic angiography, performed through antegrade access into the right common femoral artery, revealed a diffusely diseased right superficial femoral artery (SFA) and occluded P2 and P3 segment popliteal artery, with 3-vessel tibial vessel reconstitution by collaterals (Figure 5).

Antegrade crossing via the common femoral artery access and retrograde crossing of the popliteal occlusion via the anterior tibial led to the subintimal presence of the retrograde V-18 wire (Boston Scientific). A focal calcified lesion in the P2 segment of the popliteal artery prevented reentry at that level.
The decision was then made to reenter the true lumen of the SFA with an Enteer reentry wire (Medtronic) that was advanced from the retrograde route (Figure 6).

Unfortunately, at that instant, the “roadmap” feature of the x-ray equipment failed. Since there was a need for image-guided reentry into the true lumen of the right SFA, a 6 x 20 mm EverCross balloon (Medtronic) was inflated in the SFA and under fluoroscopic guidance, this balloon was punctured with the retrograde Enteer wire, thus entering the true lumen of the SFA.
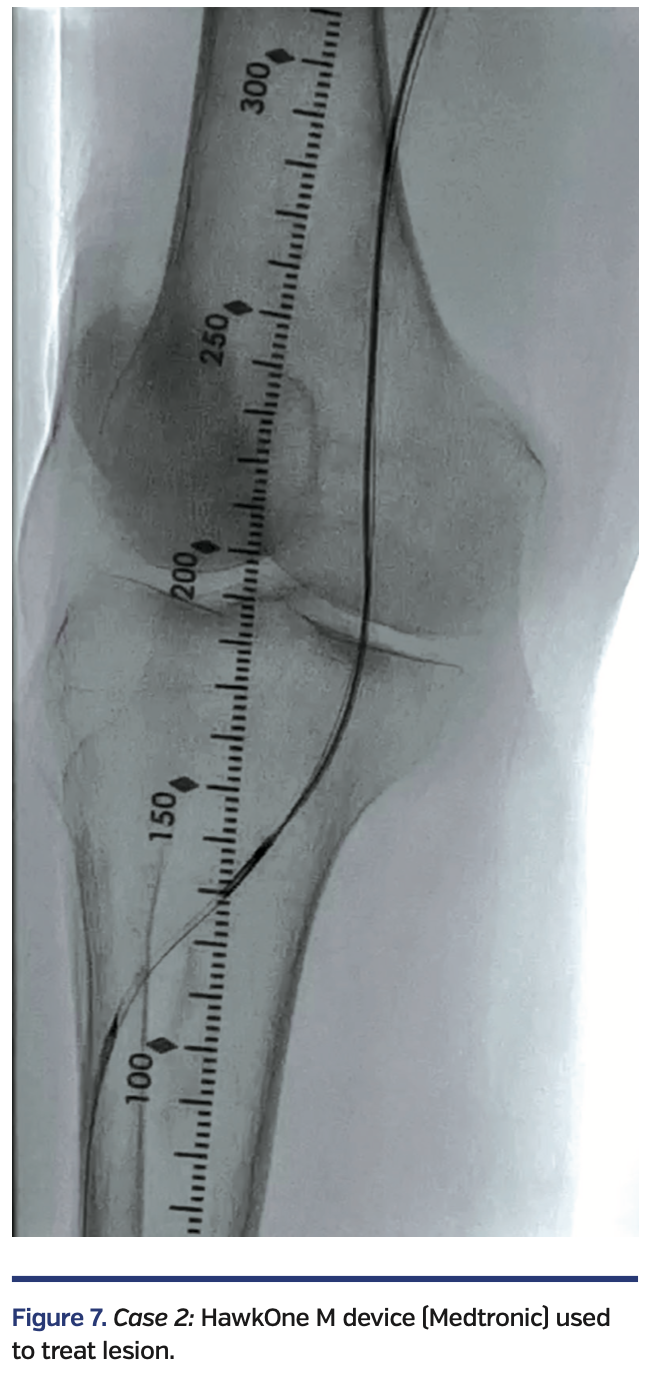
The Enteer wire was exchanged out for a V-18 wire that was subsequently externalized via the right common femoral artery sheath. Over an antegrade Fielder FC wire, intravascular ultrasound (IVUS) evaluation was performed with an .014-inch IVUS catheter, followed by directional atherectomy with a HawkOne M device (Medtronic) (Figure 7).

Sequential balloon angioplasty in the anterior tibial artery was performed with 2.0 mm and 4.0 mm Coyote balloon (Boston Scientific). Kissing balloon angioplasty of the popliteal artery, proximal anterior tibial, and tibioperoneal trunks was performed with long 3 mm balloons, followed by drug-coated balloon angioplasty of the right femoropopliteal segment with a 6 mm x 250 mm IN.PACT Admiral drug-coated balloon (Medtronic). There was restoration of normal flow in all treated segments (Figure 8), with a warm foot and a palpable dorsalis pedis artery at the end of the case.

Case 3
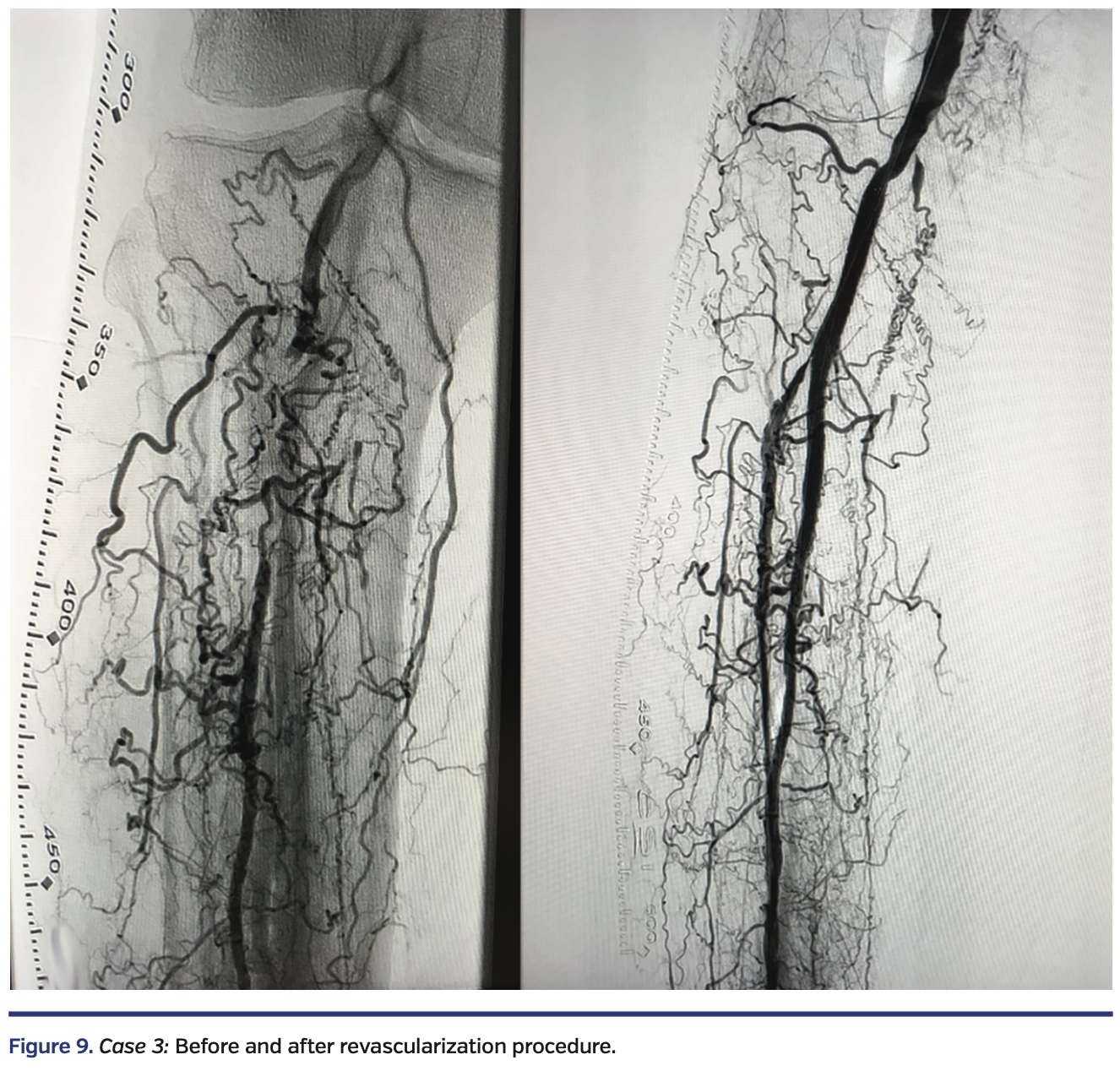
A white male in his early seventies with a history of type 2 diabetes presented with one year of claudication and a 2-month-old nonhealing ulcer at the tip of the right hallux with rest pain. Diagnostic angiography by a different physician revealed no evidence of right iliofemoral disease. The P3 segment of the right popliteal artery and the tibioperoneal trunk were occluded with recanalization of the mid peroneal artery via numerous collaterals (Figure 9, left).

Antegrade crossing of the right popliteal artery was then attempted by this physician from the left common femoral artery and subsequently, in a different attempt, via right common femoral artery access, but was unsuccessful. The patient was then referred to me. The decision was made to attempt recanalization via a tibial approach. Right peroneal artery access was obtained under fluoroscopic guidance to advance a 6 Fr Prelude Ideal sheath (Merit Medical) in a retrograde fashion (Figure 10).

A V-18 wire was advanced from the retrograde route and entered the subintimal space of the P3 segment of the popliteal artery. True lumen reentry was performed by advancing an Enteer wire with the aid of a 90 cm angled NaviCross catheter (Terumo) from the retrograde route. This wire was then exchanged out for a Fielder FC wire, followed by directional atherectomy of the right peroneal artery, right tibioperoneal trunk, and right popliteal artery, with a HawkOne M catheter advanced from the Prelude Ideal sheath, followed by balloon angioplasty with a 3.0 x 80 mm NanoCross balloon (Medtronic) and a 4.0 x 80 mm IN.PACT Admiral drug-coated balloon in the popliteal. Residual popliteal artery disease was treated with a 6 x 80 mm EverFlex self-expanding stent (Medtronic), post dilated in the proximal segment with a 6 x 40 mm and a 4 x 40 mm EverCross balloon (Medtronic) in the distal segment. To maximize blood flow and aid healing, right dorsalis pedis artery access was obtained with ultrasound and a 4 Fr merit Prelude Ideal sheath was advanced into the right distal anterior tibial artery. A knuckled Fielder FC wire was advanced into the true lumen of the right anterior tibial artery and right popliteal artery, followed by balloon angioplasty with a long 3.5 mm NanoCross balloon (Medtronic). Final angiography showed a widely patent right popliteal artery, tibioperoneal trunk, and peroneal artery (Figure 9, right). The ostium of the right anterior tibial artery was pinched, but there was normal flow in the right anterior tibial artery. There was complete resolution of the rest pain by the end of the procedure.
Discussion
Long-term survival and cost metrics in critical limb ischemia management are improved with successful endovascular revascularization procedures. Primary major amputation results in shorter survival, higher risk of subsequent major amputation, and higher healthcare costs versus revascularization.3 Use of the Medtronic HawkOne directional atherectomy system followed by drug-coated balloon angioplasty above the knee shows promising results for treating and optimizing the care of patients with severe PAD. In the DEFINITIVE AR clinical study, a multinational pilot study evaluating the effectiveness of directional atherectomy (DA) and anti-restenotic therapy (121 patients, 10 centers) demonstrated that vessel prep with DA prior to DCB resulted in increased technical success and fewer flow-limiting dissections compared to DCB alone. The DEFINITIVE AR clinical study suggests a trend towards improved patency using DA + DCB for both longer and severely calcified lesions.6 The REALITY study will further evaluate this concept with HawkOne directional atherectomy (Medtronic), followed by IN.PACT Admiral DCB (Medtronic), to treat long, calcified femoropopliteal arteries.7
This article is supported by Medtronic.
Images courtesy of Dr. Siddhartha Rao. Individual results may vary.
References
- Weitz JI, Byrne J, Clagett P, et al. Diagnosis and treatment of chronic arterial insufficiency of the lower extremities: a critical review. Circulation. 1996;94:3026-3049.
- Misra S, Shishehbor MH, Takahashi EA, et al. Perfusion assessment in critical limb ischemia: principles for understanding and the development of evidence and evaluation of devices: a scientific statement from the American Heart Association. Circulation. 2019;140(12):e657-e672. doi:10.1161/CIR.0000000000000708
- Mustapha JA, Katzen BT, Neville RF, et al. Determinants of Long-Term Outcomes and Costs in the Management of Critical Limb Ischemia: A Population-Based Cohort Study. J Am Heart Assoc. 2018;7(16):e009724. doi:10.1161/JAHA.118.009724
- Goodney PP, Travis LL, Nallamothu BK, et al. Variation in the use of lower extremity vascular procedures for critical limb ischemia [published correction appears in Circ Cardiovasc Qual Outcomes. 2012 May;5(3):e27]. Circ Cardiovasc Qual Outcomes. 2012;5(1):94-102. doi:10.1161/CIRCOUTCOMES.111.962233
- McKinsey J, Zeller T, Rocha-Singh KJ, Jaff MR, Garcia LA; DEFINITIVE LE Investigators. Lower extremity revascularization using directional atherectomy: 12-month prospective results of the DEFINITIVE LE study. JACC Cardiovasc Interv. August 2014;7(8):923-933.
- Zeller T, Langhoff R, Rocha-Singh KJ, et al. Directional Atherectomy Followed by a Paclitaxel-Coated Balloon to Inhibit Restenosis and Maintain Vessel Patency: Twelve-Month Results of the DEFINITIVE AR Study. Circ Cardiovasc Interv. 2017;10(9):e004848. doi:10.1161/CIRCINTERVENTIONS.116.004848
- DiRectional AthErectomy + Drug CoAted BaLloon to Treat Long, CalcifIed FemoropopliTeal ArterY Lesions (REALITY): https://clinicaltrials.gov/ct2/show/NCT02850107
 Disclosure: Dr. Rao reports he is a consultant to Medtronic, CSI, and Intact Vascular.
Disclosure: Dr. Rao reports he is a consultant to Medtronic, CSI, and Intact Vascular.
Dr. Siddhartha Rao can be contacted at srao@apcnc.org.
For US Audiences Only
HawkOne™ Directional Atherectomy System
The HawkOne™ peripheral directional atherectomy system is intended for use in atherectomy of the peripheral vasculature. The HawkOne™ catheter is indicated for use in conjunction with the SpiderFX™ embolic protection device in the treatment of severely calcified lesions. The HawkOne™ catheter is not intended for use in the coronary, carotid, iliac or renal vasculature.
IN.PACT™ Admiral™ Drug Coated PTA Balloon Catheter Brief Statement
FTSOP113326-32 Rev. 1H
Indications for Use:
The IN.PACT™ Admiral™ Paclitaxel-coated PTA Balloon Catheter is indicated for percutaneous transluminal angioplasty, after appropriate vessel preparation, of de novo, restenotic, or in-stent restenotic lesions with lengths up to 360 mm in superficial femoral or popliteal arteries with reference vessel diameters of 4-7 mm.
Contraindications
The IN.PACT Admiral DCB is contraindicated for use in:
• Coronary arteries, renal arteries, and supra-aortic/cerebrovascular arteries
• Patients who cannot receive recommended antiplatelet and/or anticoagulant therapy
• Patients judged to have a lesion that prevents complete inflation of an angioplasty balloon or proper placement of the delivery system
• Patients with known allergies or sensitivities to paclitaxel
• Women who are breastfeeding, pregnant or are intending to become pregnant or men intending to father children. It is unknown whether paclitaxel will be excreted in human milk and whether there is a potential for adverse reaction in nursing infants from paclitaxel exposure.
Warnings
• A signal for increased risk of late mortality has been identified following the use of paclitaxel-coated balloons and paclitaxel-eluting stents for femoropopliteal arterial disease beginning approximately 2-3 years post-treatment compared with the use of non-drug coated devices. There is uncertainty regarding the magnitude and mechanism for the increased late mortality risk, including the impact of repeat paclitaxel-coated device exposure. Physicians should discuss this late mortality signal and the benefits and risks of available treatment options with their patients.
• Use the product prior to the Use-by Date specified on the package.
• Contents are supplied sterile. Do not use the product if the inner packaging is damaged or opened.
• Do not use air or any gaseous medium to inflate the balloon. Use only the recommended inflation medium (equal parts contrast medium and saline solution).
• Do not move the guidewire during inflation of the IN.PACT Admiral DCB.
• Do not exceed the rated burst pressure (RBP). The RBP is 14 atm (1419 kPa) for all balloons except the 200 and 250 mm balloons. For the 200 and 250 mm balloons the RBP is 11 atm (1115 kPa). The RBP is based on the results of in vitro testing. Use of pressures higher than RBP may result in a ruptured balloon with possible intimal damage and dissection.
• The safety and effectiveness of using multiple IN.PACT Admiral DCBs with a total drug dosage exceeding 34,854 µg of paclitaxel in a patient has not been clinically evaluated.
Precautions
• This product should only be used by physicians trained in percutaneous transluminal angioplasty (PTA).
• This product is designed for single patient use only. Do not reuse, reprocess, or resterilize this product. Reuse, reprocessing, or resterilization may compromise the structural integrity of the device and/or create a risk of contamination of the device, which could result in patient injury, illness, or death.
• Assess risks and benefits before treating patients with a history of severe reaction to contrast agents.
• The safety and effectiveness of the IN.PACT Admiral DCB used in conjunction with other drug-eluting stents or drug-coated balloons in the same procedure or following treatment failure has not been evaluated.
• The extent of the patient’s exposure to the drug coating is directly related to the number of balloons used. Refer to the Instructions for Use (IFU) for details regarding the use of multiple balloons and paclitaxel content.
• The use of this product carries the risks associated with percutaneous transluminal angioplasty, including thrombosis, vascular complications, and/or bleeding events
• Vessel preparation using only pre-dilatation was studied in the clinical study. Other methods of vessel preparation, such as atherectomy, have not been studied clinically with IN.PACT Admiral DCB.
• This product is not intended for the expansion or delivery of a stent.
Potential Adverse Effects
The potential adverse effects (e.g. complications) associated with the use of the device are: abrupt vessel closure; access site pain; allergic reaction to contrast medium, antiplatelet therapy, or catheter system components (materials, drugs, and excipients); amputation/loss of limb; arrhythmias; arterial aneurysm; arterial thrombosis; arteriovenous (AV) fistula; death; dissection; embolization; fever; hematoma; hemorrhage; hypotension/hypertension; inflammation; ischemia or infarction of tissue/organ; local infection at access site; local or distal embolic events; perforation or rupture of the artery; pseudoaneurysm; renal insufficiency or failure; restenosis of the dilated artery; sepsis or systemic infection; shock; stroke; systemic embolization; vessel spasms or recoil; vessel trauma which requires surgical repair.
Potential complications of peripheral balloon catheterization include, but are not limited to the following: balloon rupture; detachment of a component of the balloon and/or catheter system; failure of the balloon to perform as intended; failure to cross the lesion.
Although systemic effects are not anticipated, potential adverse events that may be unique to the paclitaxel drug coating include, but are not limited to: allergic/immunologic reaction; alopecia; anemia; gastrointestinal symptoms; hematologic dyscrasia (including leucopenia, neutropenia, thrombocytopenia); hepatic enzyme changes; histologic changes in vessel wall, including inflammation, cellular damage, or necrosis; myalgia/arthralgia; myelosuppression; peripheral neuropathy.
Refer to the Physician’s Desk Reference for more information on the potential adverse effects observed with paclitaxel. There may be other potential adverse effects that are unforeseen at this time.
Please reference appropriate product Instructions for Use for a detailed list of indications, warnings, precautions and potential adverse effects. This content is available electronically at www.manuals.medtronic.com.
Enteer™ Re-entry Guidewire
Indications for Use: The Enteer™ Re-entry Guidewire is intended to facilitate placement of balloon dilatation catheters or other intravascular devices during percutaneous transluminal angioplasty (PTA). The Enteer Guidewire is not to be used in cerebral blood vessels. When used as part of the Peripheral System, the Enteer Guidewire is indicated for use to facilitate the intraluminal placement of conventional guidewires beyond stenotic peripheral lesions (including chronic total occlusions) prior to placement of other interventional devices.
EverCross™ 0.035” PTA Balloon Catheter
Indications for Use: The EverCross™ 0.035” OTW PTA Dilatation Catheter is intended to dilate stenoses in the iliac, femoral, iliofemoral, popliteal, infrapopliteal, and renal arteries, and for the treatment of obstructive lesions of native or synthetic arteriovenous dialysis fistulae. This device is also indicated for stent post-dilatation in the peripheral vasculature.
EverFlex™ Self-expanding Peripheral Stent System
Indication: The EverFlex™ Self-Expanding Peripheral Stent System is intended to improve luminal diameter in the treatment of symptomatic de novo or restenotic lesions up to 180 mm in length in the native superficial femoral artery and/or proximal popliteal arteries with reference vessel diameters ranging from 4.5 mm-7.5 mm. The EverFlex Self-Expanding Peripheral Stent System is indicated for improving luminal diameter in patients with atherosclerotic disease of the common and/or external iliac arteries up to and including 100 mm in length, with a reference vessel diameter of 4.5 mm-7.5 mm. The Protégé EverFlex Self-expanding Biliary Stent System is intended as a palliative treatment of malignant neoplasms in the biliary tree.
Contraindications: Use of the EverFlex™ Self-Expanding Peripheral Stent System is contraindicated in patients with known hypersensitivity to nickel titanium and in patients contraindicated for anticoagulant and/or antiplatelet therapy, patients who are judged to have a lesion that prevents complete inflation of an angioplasty balloon or proper placement of the stent or stent delivery system.
Potential Adverse Events: Potential adverse events which may be associated with the use of a stent in the SFA and proximal popliteal arteries include, but are not limited to: Allergic reaction, Amputation, Artery perforation or rupture, Bleeding requiring transfusion, Infection, Pseudoaneurysm, Restenosis, Stent collapse or fracture, Stent migration, Surgical or endovascular intervention, Thrombosis/occlusion of the stent.
See the Instructions for Use provided with the product for a complete list of warnings, precautions, adverse events, and device information.
NanoCross™ Elite 0.014” PTA Balloon Catheter
Indications for Use: The NanoCross™ Elite 0.014” Over-the-Wire PTA Balloon Dilatation Catheter is intended to dilate stenoses in the iliac, femoral, iliofemoral, popliteal, infra-popliteal, and renal arteries, and for the treatment of obstructive lesions of native or synthetic arteriovenous dialysis fistulae. This device is also indicated for stent post-dilatation in the peripheral vasculature.
UC202103111 EN © 2020 Medtronic. All brands are trademarks of their respective owners