Invasive Hemodynamic Correlates of Multiple Bedside Cardiovascular Eponyms in a Patient With Advanced Heart Failure
Identifying specific patterns of cardiopulmonary abnormalities can provide independent prognostic information that may help clinicians in their decision-making when assessing a patient’s severity of illness. In this concise manuscript, we reacquaint the readers with prognostically important invasive hemodynamic correlates of classical cardiovascular eponyms seen in heart failure: Traube’s pulse, Kussmaul physiology, and Cheyne-Stokes respiration. Seeking out and noting such visual displays of hemodynamic and respiratory abnormalities, and linking them to eponyms, not only increases enjoyment on rounds, but also may help renew interest in the bedside exam.
Case and Findings
A 46-year-old man was transferred to our facility for consideration of advanced heart failure therapies after he was diagnosed with new-onset systolic heart failure 1 month prior to presentation. On arrival,he was on a continuous infusion of dobutamine at 5 mcg/kg/min, and had acute renal failure and ischemic hepatitis.
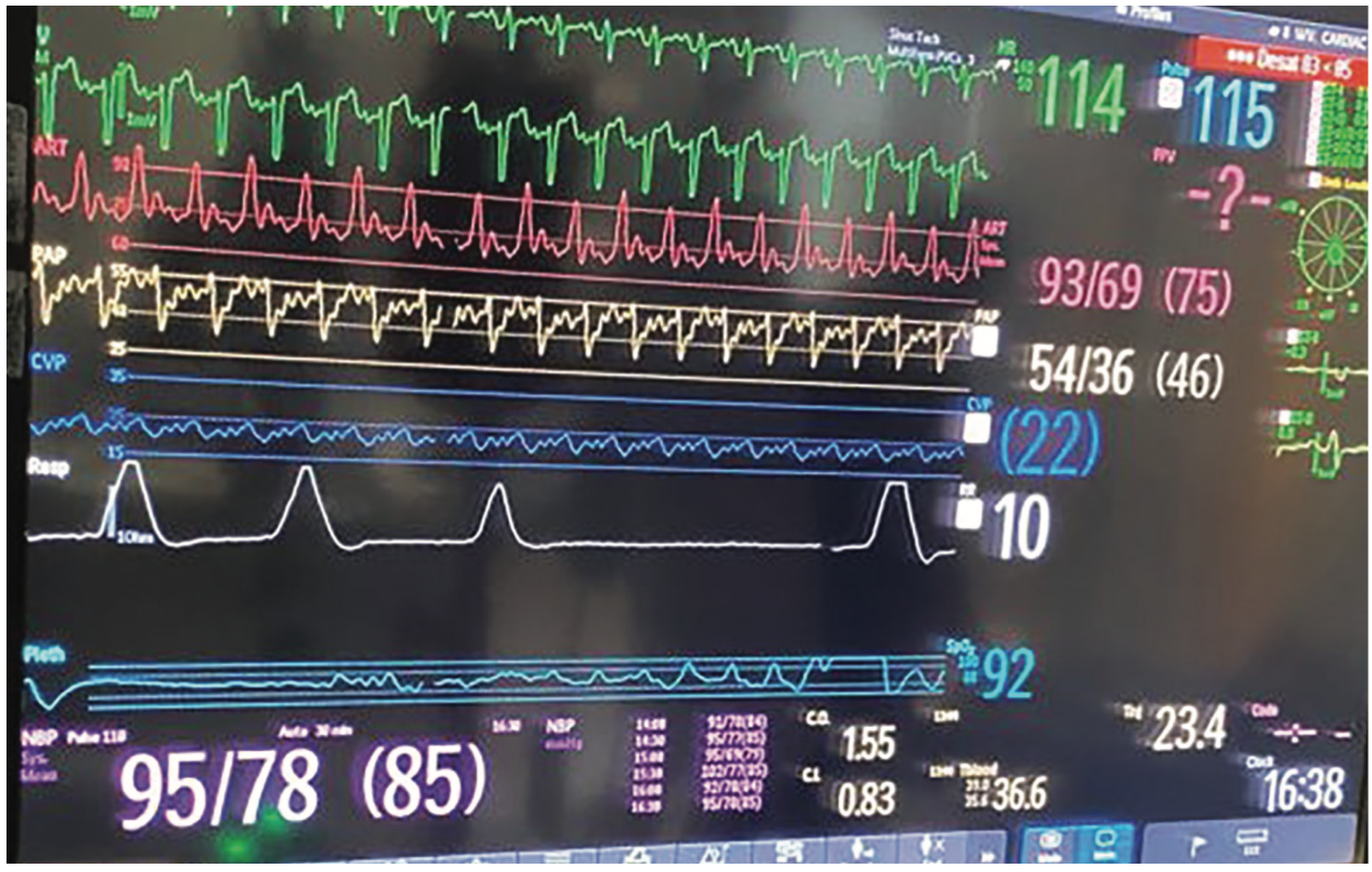
Right heart catheterization was notable for elevated right and left heart pressures, and severely reduced cardiac output (Figure 1). Of interest, his hemodynamic tracings demonstrated several findings known to be associated with an increased mortality in heart failure and which have received an eponym:
(1) Traube’s pulse, also known as pulsus alternans1, was noted on the arterial (Figure 1, red), and pulmonary artery waveforms (Figure 1, yellow), corresponding to severely impaired bi-ventricular systolic function (Video 1). There are two proposed mechanisms for Traube’s pulse. The first mechanism involves the Frank-Starling relationship, whereby beat-to-beat variations in end-systolic and end-diastolic volume result in variation of the contractile force generated. The second mechanism relates to abnormal intracellular calcium handling resulting in fluctuations in myocardial contractile force, and therefore, forward stroke volume.
Video 1. Cardiac magnetic resonance imaging showing severe biventricular systolic dysfunction.
(2) Kussmaul physiology2 was evident in this patient when comparing the central venous pressure (CVP) tracing (Figure 1, navy blue) to the plethysmograph waveform (Figure 1, white), as the right atrial pressure (RAP) did not decrease during inspiration. In some cases, there can be a paradoxical inspiratory rise in the RAP. Under normal circumstances, the right ventricle (RV) should be able to accommodate the increased venous return to the right heart that occurs during inspiration. However, this is not the case if the RV is noncompliant or constricted (as is the case in constrictive pericarditis). In addition to RV and pericardial pathology, abnormal right heart-pulmonary vascular interaction has also been proposed as a contributor to Kussmal physiology.
(3) Cheyne-Stokes breathing (Figure 1, white) composed of periodic hyperpnea, hypopnea, and apnea reflecting unstable ventilatory control due to prolonged circulation time.3 In heart failure, pulmonary congestion resulting in impaired oxygen diffusion across the alveolar membrane and low cardiac output contribute to an increased transit time between the lungs and brain chemoreceptors, resulting in the Cheyne-Stokes breathing pattern. Furthermore, during periods of hyperpnea, sympathetic nerve activity is increased, resulting in increases in blood pressure and heart rate that increase the risk of ischemia, arrhythmia, and cardiovascular mortality.
Conclusion
We believe that seeking out and noting such visual displays of hemodynamic and respiratory abnormalities, and linking them to eponyms, not only increases enjoyment, but also may help renew interest in the bedside exam.4 Furthermore, identifying these patterns of hemodynamic waveforms can provide independent prognostic information, which may help clinicians in their decision-making when assessing a patient’s severity of illness.
Disclosures: The authors report no conflicts of interest regarding the content herein.
The authors can be contacted via Faris G. Araj, MD, at faris.araj@utsouthwestern.edu
References
1. Szymanski P, Lipczynska M, Klisiewicz A, Hoffman P. “Like a sound and its echo”. Biventricular pulsus alternans. Heart. 2014 Jan; 100(1): 83, 90. doi: 10.1136/heartjnl-2013-304922
2. Nadir AM, Beadle R, Lim HS. Kussmaul physiology in patients with heart failure. Circ Heart Fail. 2014 May; 7(3): 440-447. doi: 10.1161/CIRCHEARTFAILURE.113.000830
3. Puri P, Mehra A, Elkayam U. Cheyne-Stokes respiration and cardiac hemodynamics in heart failure. Catheter Cardiovasc Interv. 2008 Oct 1; 72(4): 581-585. doi: 10.1002/ccd.21636
4. Ma I, Tierney LM. Name that murmur--eponyms for the astute auscultician. N Engl J Med. 2010 Nov 25; 363(22): 2164-2168. doi: 10.1056/NEJMon1006947










