Intravascular Lithotripsy in an Underexpanded Stent Due to a Severely Calcified Distal Left Main Lesion
Abstract
Severe coronary artery calcification is one of the most challenging scenarios encountered during percutaneous coronary intervention. Coronary artery calcification is associated with higher rates of stent failure and is linked to poor outcomes in percutaneous coronary intervention. Many technologies like noncompliant balloons, high pressure balloons, cutting/scoring balloons, and laser and atherectomy procedures have been developed to manage such dense, calcific lesions, but these technologies are not devoid of complications. Intravascular lithotripsy is a relatively new technology that dispenses circumferential mechanical energy to fracture the calcium and assist in stent expansion. Here, we present a case of a distal left main stent underexpansion that was successfully treated with intravascular lithotripsy.
Introduction
Severe coronary artery calcification (CAC) poses a great challenge for percutaneous coronary intervention (PCI). CAC is an independent predictor for stent failure and major adverse cardiac events.1,2 Heavy calcification leads to stent underexpansion and eventually, stent failure.3 Multiple technologies including high-pressure noncompliant (NC) balloons, cutting/scoring balloons, and rotational/orbital/laser atherectomy have been used to treat calcific lesions, but are associated with a high procedural complication rate.4 The effectiveness of these tools is further reduced in cases of deep, thick, and circumferential calcific lesions. Intravascular lithotripsy (IVL) modifies thick, deep, circumferential calcified plaque5,6 by employing the circumferential distribution of the high-pressure sonic waves to fracture both superficial and deep calcium.4 We present what we believe to be the first case using IVL in the distal left main (LM) for stent underexpansion due to heavy calcification.
Case Description
An 88-year-old gentleman with a past medical history of hypertension, chronic kidney disease with a creatinine of 2 mg/dL, peripheral vascular disease (complete left internal carotid artery occlusion and moderate left internal carotid artery stenosis), and hyperlipidemia presented to the clinic with progressive, lifestyle-limiting angina despite optimal medical therapy. Four years ago, he presented with symptomatic severe aortic stenosis and later was found to have severe ostial left main (LM) and ostial left circumflex (LCX) disease that was managed with a drug-eluting stent (DES) (a 3 mm x 32 mm Xience Alpine [Abbott Vascular]). His stent procedure was closely followed by transcatheter aortic valve replacement (TAVR) with a 26 mm Sapien 3 (Edwards Lifesciences) for severe aortic valve stenosis (Video 1).
Video 1. Transcatheter aortic valve replacement with a 26 mm Sapien 3 (Edwards Lifesciences).
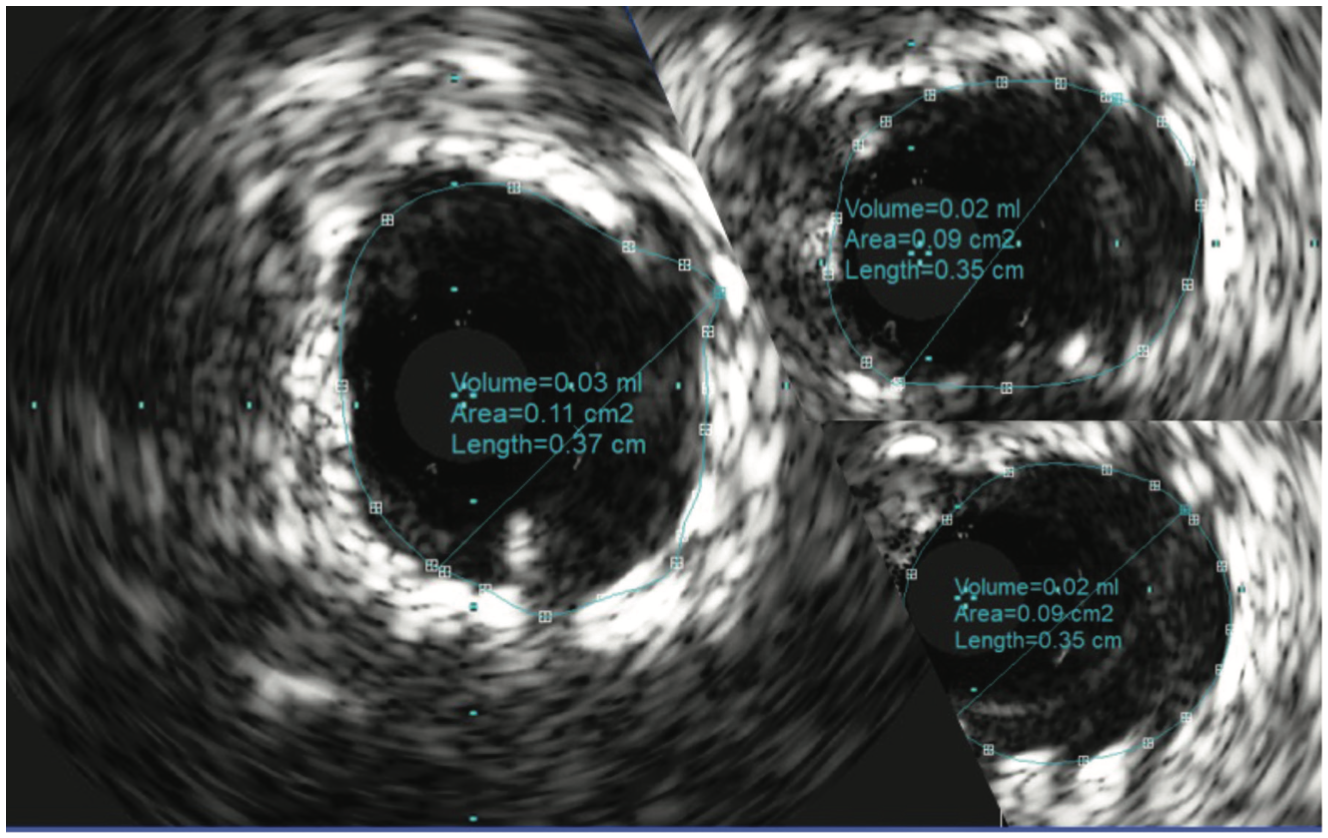
The patient’s electrocardiogram showed sinus rhythm with left bundle branch block and echocardiography demonstrated a normal left ventricular ejection fraction with a transvalvular aortic mean gradient of 10 mmHg. On further evaluation, his nuclear stress test showed severe anterior ischemia. A review of his previous angiograms demonstrated an underexpanded stent in the distal LM that was potentially constrained by a circumferential calcific lesion (Figure 1). Intravascular ultrasound (IVUS) and atherectomy were not utilized during the index procedure. The patient underwent cardiac catheterization via the right femoral approach, which demonstrated severe in-stent restenosis (ISR) of the distal LM due to an underexpanded/undersized stent (Figure 2), confirmed on assessment of the non-contrast chest imaging and IVUS, with a minimum stent area (MSA) of 4 mm2 (Figure 3). Given the patient was not a surgical candidate, further percutaneous management was elected as the best management strategy since the patient had refractory angina despite medical therapy. Mechanical support with large-bore access was not feasible, given the vascular disease in his lower extremities had worsened, and with severe carotid disease, the patient was not a candidate for axillary large-bore devices. He was pre-treated with aspirin and clopidogrel before the PCI.
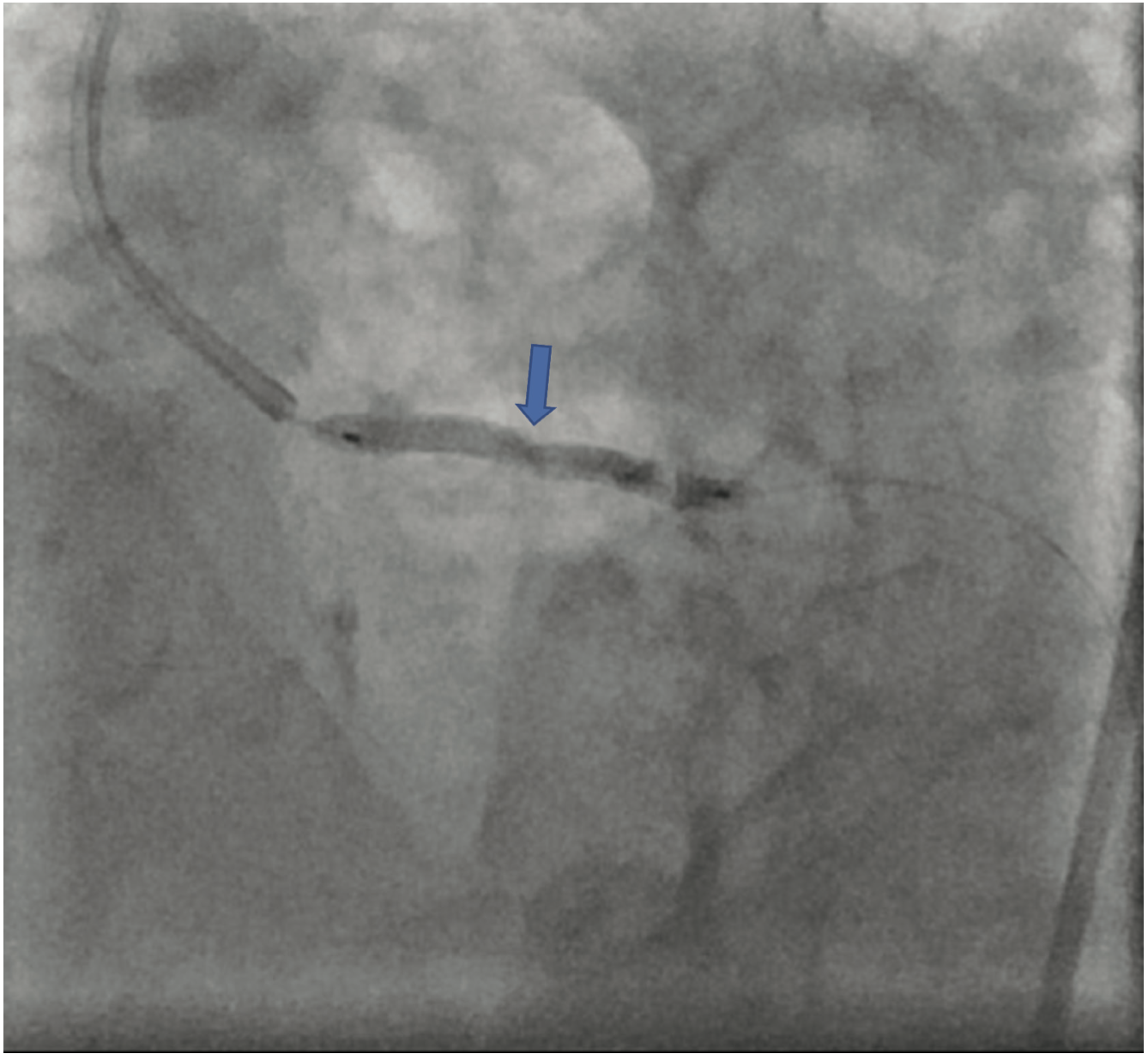
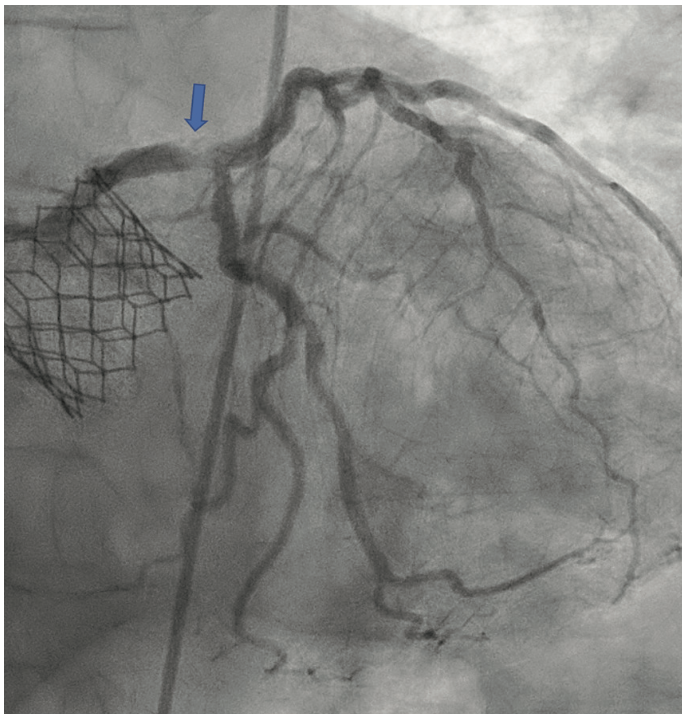
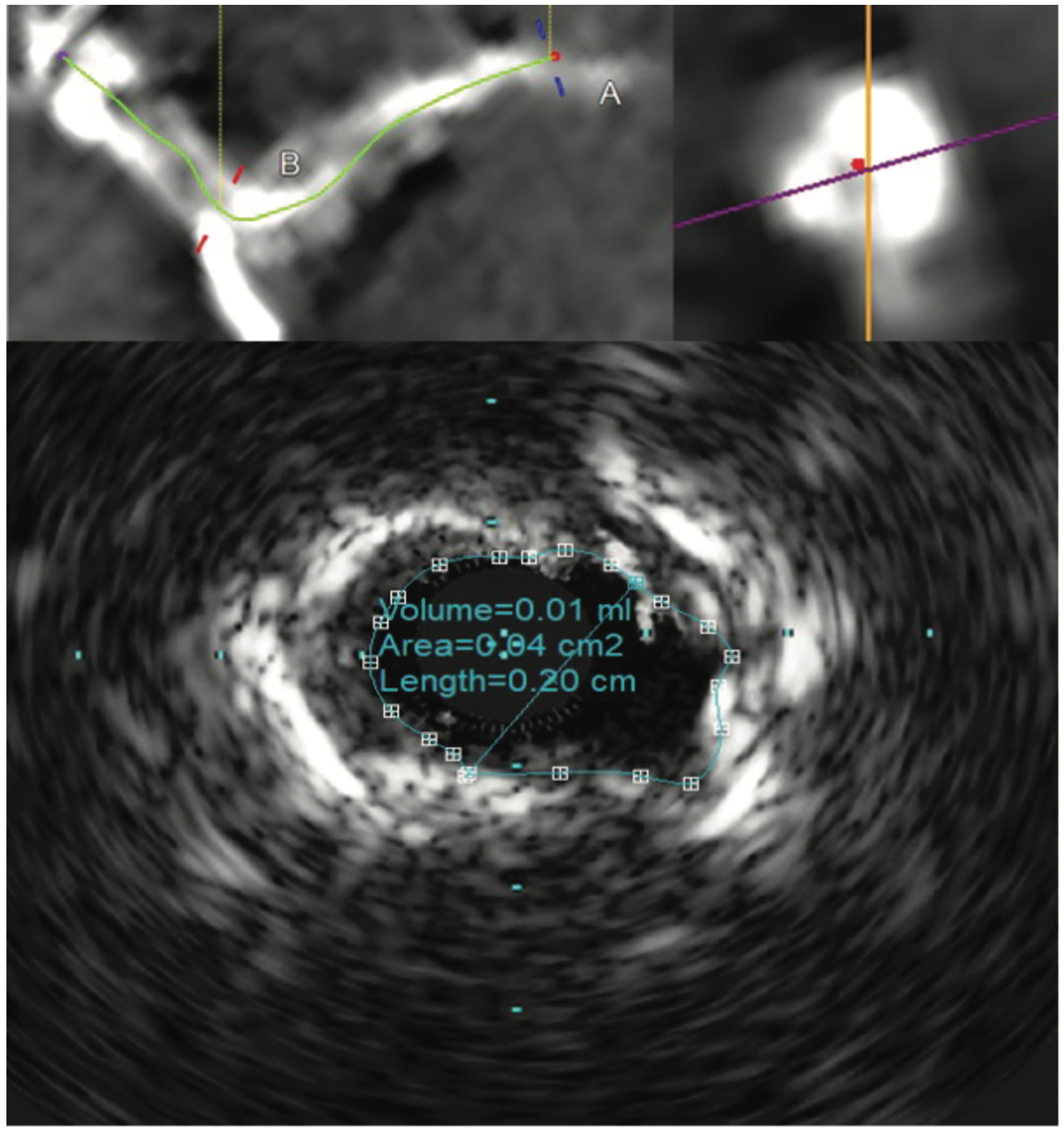
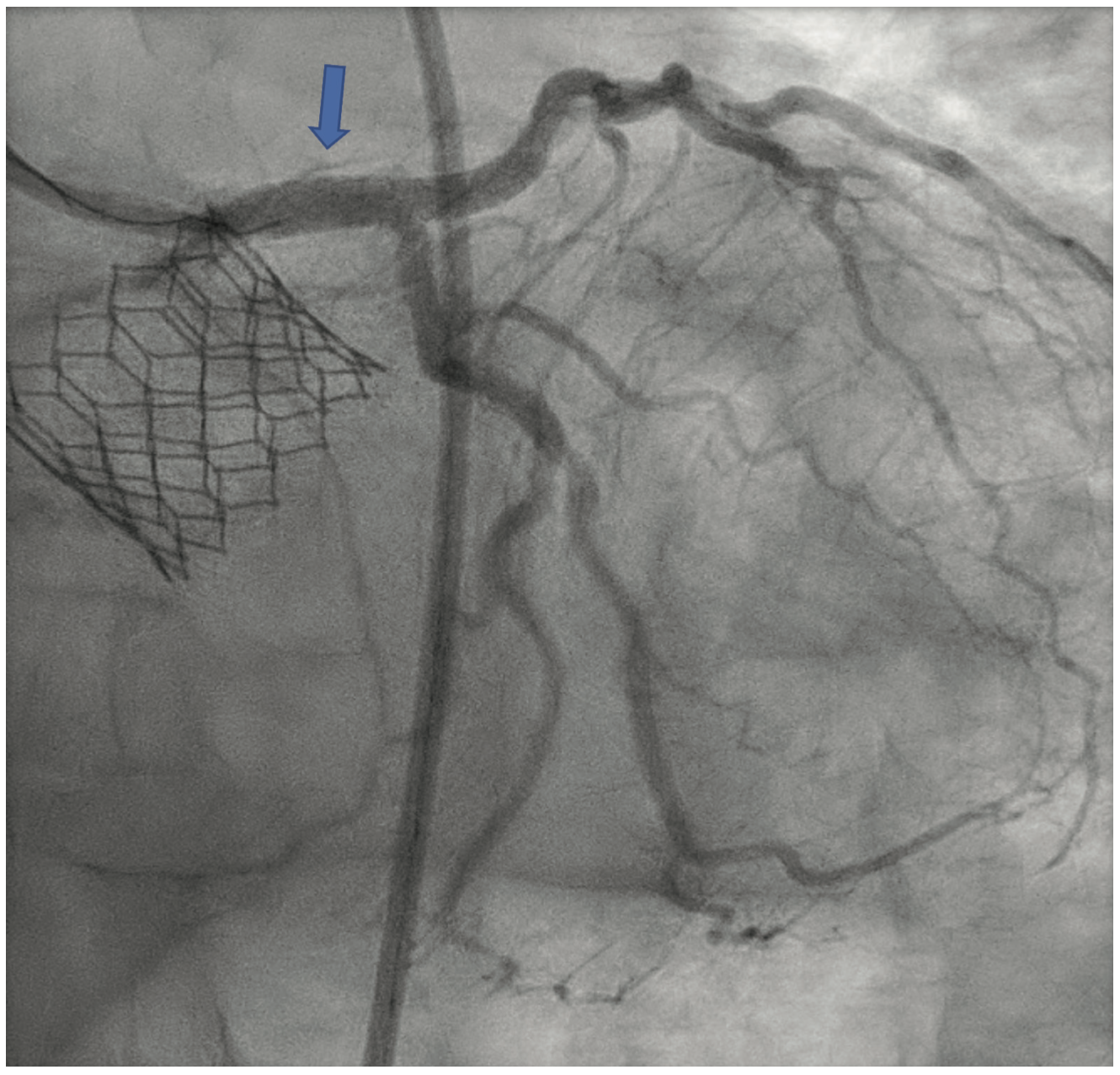
The LM was engaged with a 7 French (F) XB 3.5 guide catheter (Cordis Corporation). A workhorse coronary wire was advanced to the distal left anterior descending (LAD) coronary artery and another workhorse wire was advanced to the distal obtuse marginal. Serial inflations with 3.5 mm x 20 mm NC balloon were performed at 20 atmospheres (atm) in the distal LM. A kissing balloon inflation (KBI) was done using two 3.5 mm x 12 mm NC balloons at 12 atm. The ostium of the left circumflex (LCX) and distal LM was further prepared using a 4 mm x 10 mm Wolverine cutting balloon (Boston Scientific) at 12 atm. The distal LM lesion did not yield, despite multiple balloon inflations. Hence, a 4 mm x 12 mm IVL balloon (Shockwave Medical) was used at 4 atm for 40 pulses. These inflations had to be supported with intravenous norepinephrine boluses. Later, a 4 mm x 12 mm NC balloon was used and the result showed adequate expansion across the LCX ostium. A double-kissing culotte was completed and the proximal LAD was stented with a 3.5 mm x 24 mm Synergy Megatron (Boston Scientific) at 12 atm. Proximal optimization technique (POT) was performed using a 5 mm x 12 mm NC balloon at 20 atm. The LCX was re-crossed using another workhorse wire and the jailed LCX wire was removed. The side struts were opened using a 2 mm x 12 mm semi-compliant balloon, followed by KBI with two 4 mm x 12 mm NC balloons at 12 atm. Re-POT was done using 5.5 mm x 12 mm NC balloon at 22 atm with an excellent result (Figure 4). The final IVUS demonstrated a MSA of 11 mm2 in the distal LM, and 9 mm2 MSA was attained in the ostium of the LAD and LCX (Figure 5). Total contrast used in the procedure was 30 mL, as IVUS was used significantly during the PCI in view of the complex lesion, as well as the patient’s pre-existing renal impairment. He was discharged the next day after post PCI hydration with dual antiplatelet therapy for six months and he had no change in creatinine at the end of one week. At four weeks, the patient is well without any angina and is asymptomatic from a cardiac perspective.
Discussion
In this case, we have described the successful use of IVL to manage an underexpanded stent due to severe CAC in the distal LM. To the best of our knowledge, this is the first case that has reported the use of IVL for stent underexpansion in the distal LM. IVL is currently off-label for in-stent restenosis, but is an invaluable tool in the arsenal. The index procedure was challenging, given the patient had a severe aortic stenosis and a complex calcific left main lesion. It is likely the operators were not able to utilize a full arsenal of atherectomy devices. Previous reports have been described wherein a balloon aortic valvuloplasty was followed by Protected PCI.7 However, this strategy can potentially result in major vascular complications.8 Our case was performed at an outreach facility where there was no surgical backup. IVL, being a safe and effective procedure with minimal complication rates, can be considered in hospitals without surgical reserves and possibly in ambulatory surgical centers. It is an excellent alternative to staged atherectomy procedures.
The role of NC balloon dilations, cutting and scoring balloons, intracoronary laser, rotational atherectomy, and orbital atherectomy in ISR has been previously described, but each tool comes with its own, respective limitations.9,10 NC balloon dilations create inadequate force to fracture the extrinsic calcium and provide inadequate stent expansion despite the high pressure.5 Atherectomy devices are associated with drastic complications such as slow-flow/no-flow phenomena, dissection, and perforation.5 To help overcome these shortcomings, a novel, percutaneous technique used to treat deep calcium, IVL (Shockwave Medical), has been developed.11 The IVL system is comprised of a single-use catheter with multiple lithotripsy emitters that are encompassed in a balloon. IVL works by releasing high-speed, sonic pressure waves in a circumferential field that crosses the vessel wall soft tissue, selectively affecting the subendothelial calcium, fracturing the calcium deposits, and altering the vessel compliance.12
IVL provides multiple advantages over specialty balloons or atherectomy techniques.5 It does not require any learning curve for operators comfortable with performing plain angioplasty, as it is similar to conventional, catheter-based PCI. As a balloon-based technique, the risk of distal embolization is lower compared to the atherectomy devices. In addition, calcified plaque modification is not based on guidewire bias, as energy is uniformly delivered over the lithotripsy emitter to fracture calcium.5 Further, where conventional balloon techniques work on static barometric pressure, IVL dispenses ultrashort pulses of high-intensity acoustic energy circumferentially, which, in turn, results in modification of the calcified plaque.5 Protection of side branches can easily be achieved in IVL by using a guidewire, in contrast to atherectomy procedures where wire entrapment or amputation can occur.5
Tizón-Marcos et al reported balloon rupture from a fractured calcium segment as a complication of IVL, and continuous fluoroscopy during the procedure is advised for early detection of the balloon leakage.13 The safety and efficacy of IVL has also been demonstrated in the Disrupt CAD I study in 60 patients with severe CAC prior to DES implantation.6 The trial demonstrated successful stent delivery in all 60 patients, and device and clinical success in 59 and 57 patients, respectively. There were no major complications such as perforation, embolization, slow flow or no-reflow reported.6 Disrupt CAD II further confirmed the safety of IVL, showing high procedural success without any dissection, perforation, acute vessel closure or slow/no re-flow phenomena.5
CAC is attributed with a higher risk of stent underexpansion, stent thrombosis, and ischemic target lesion revascularization.14 Numerous cases have been reported in the literature that describe the use of IVL for stent underexpansion due to CAC.12,13,15-27 We are aware of one case reporting a procedural complication of balloon rupture13, but the remainder witnessed success without any drawbacks. The target vessel in these cases included either the left anterior descending artery, right coronary artery, left circumflex, or ramus intermedius, but our case was unique, as the intervention involved the left main coronary artery. Our case also demonstrated a successful IVL procedure without any periprocedural complications and excellent follow-up results.
Conclusion
IVL is a promising technology to treat stent underexpansion secondary to extrinsic calcification. It serves as a good alternative to existing atherectomy devices or balloon techniques with minimal complications, especially in cases of circumferential calcification. Further larger, randomized studies are required to evaluate the effectiveness and any complications related to this procedure.
Disclosures: Dr. Agrawal, Dr. Sarraf, and Dr. Nagaraja report no conflicts of interest regarding the content herein.
The authors can be contacted via Dr. Vinayak Nagaraja at nagaraja.vinayak@mayo.edu
References
1. Madhavan MV, Tarigopula M, Mintz GS, et al. Coronary artery calcification: pathogenesis and prognostic implications. J Am Coll Cardiol. 2014 May 6; 63(17): 1703-1714. doi: 10.1016/j.jacc.2014.01.017
2. Généreux P, Redfors B, Witzenbichler B, et al. Two-year outcomes after percutaneous coronary intervention of calcified lesions with drug-eluting stents. Int J Cardiol. 2017 Mar 15; 231: 61-67. doi: 10.1016/j.ijcard.2016.12.150
3. Généreux P, Madhavan MV, Mintz GS, et al. Ischemic outcomes after coronary intervention of calcified vessels in acute coronary syndromes. Pooled analysis from the HORIZONS-AMI (Harmonizing Outcomes With Revascularization and Stents in Acute Myocardial Infarction) and ACUITY (Acute Catheterization and Urgent Intervention Triage Strategy) TRIALS. J Am Coll Cardiol. 2014 May 13; 63(18): 1845-1854. doi: 10.1016/j.jacc.2014.01.034
4. Forero MNT, Daemen J. The coronary intravascular lithotripsy system. Interv Cardiol. 2019 Nov 18; 14(3): 174-181. doi: 10.15420/icr.2019.18.R1
5. Ali ZA, Nef H, Escaned J, et al. Safety and effectiveness of coronary intravascular lithotripsy for treatment of severely calcified coronary stenoses: the Disrupt CAD II study. Circ Cardiovasc Interv. 2019 Oct; 12(10): e008434. doi: 10.1161/CIRCINTERVENTIONS.119.008434.
6. Brinton TJ, Ali ZA, Hill JM, et al. Feasibility of Shockwave coronary intravascular lithotripsy for the treatment of calcified coronary stenoses. Circulation. 2019 Feb 5; 139(6): 834-836. doi: 10.1161/CIRCULATIONAHA.118.036531
7. Diaz Quintero L, Gajo E, Guerrero M, et al. Balloon aortic valvuloplasty followed by Impella®-assisted left main coronary artery percutaneous coronary intervention in patients with severe aortic stenosis as a bridge to transcatheter aortic valve replacement. Cardiovasc Revasc Med. 2021 Jan; 22: 16-21. doi: 10.1016/j.carrev.2020.06.003
8. Amin AP, Spertus JA, Curtis JP, et al. The evolving landscape of Impella use in the United States among patients undergoing percutaneous coronary intervention with mechanical circulatory support. Circulation. 2020 Jan 28; 141(4): 273-284. doi: 10.1161/CIRCULATIONAHA.119.044007
9. Sharma SK, Duvvuri S, Dangas G, et al. Rotational atherectomy for in-stent restenosis: acute and long-term results of the first 100 cases. J Am Coll Cardiol. 1998 Nov; 32(5): 1358-1365. doi: 10.1016/s0735-1097(98)00382-9
10. Neupane S, Basir M, Tan C, et al. Feasibility and safety of orbital atherectomy for the treatment of in-stent restenosis secondary to stent under-expansion. Catheter Cardiovasc Interv. 2021 Jan 1;97(1):2-7. doi: 10.1002/ccd.28675
11. Intravascular Lithotripsy (IVL). Shockwave Medical. Accessed February 15, 2022. Available online at: https://shockwavemedical.com/technology/intravascular-lithotripsy-ivl/
12. McCutcheon K, Bennett J, Adriaenssens T. Double stent fracture and in-stent restenosis due to nodular calcification treated with Shockwave coronary intravascular lithotripsy. Catheter Cardiovasc Interv. 2020 Oct 1; 96(4): E455-E457. doi: 10.1002/ccd.28732
13. Tizón-Marcos H, Rodríguez-Costoya I, Tevar C, Vaquerizo B. Intracoronary lithotripsy for calcific neoatherosclerotic in-stent restenosis: a case report. Eur Heart J Case Rep. 2020 Jun 9; 4(4): 1-4. doi: 10.1093/ehjcr/ytaa117
14. Kassimis G, Raina T, Kontogiannis N, et al. How should we treat heavily calcified coronary artery disease in contemporary practice? From atherectomy to intravascular lithotripsy. Cardiovasc Revasc Med. 2019 Dec; 20(12): 1172-1183. doi: 10.1016/j.carrev.2019.01.010
15. Wong B, El-Jack S, Newcombe R, et al. Shockwave intravascular lithotripsy of calcified coronary lesions in ST-elevation myocardial infarction: first-in-man experience. J Invasive Cardiol. 2019 May; 31(5): E73-E75.
16. Urbano Carrillo CA, Cano García M, Muñoz Jiménez LD. Coronary lithoplasty in the treatment of stent underexpansion. Rev Esp Cardiol (Engl Ed). 2020 May; 73(5): 406. English, Spanish. doi: 10.1016/j.rec.2019.04.009
17. Alfonso F, Bastante T, Antuña P, et al. Coronary lithoplasty for the treatment of undilatable calcified de novo and in-stent restenosis lesions. JACC Cardiovasc Interv. 2019 Mar 11; 12(5): 497-499. doi: 10.1016/j.jcin.2018.12.025
18. Salazar C, Escaned J, Tirado G, Gonzalo N. Undilatable calcific coronary stenosis causing stent underexpansion and late stent thrombosis: a complex scenario successfully managed with intravascular lithotripsy. JACC Cardiovasc Interv. 2019 Aug 12; 12(15): 1510-1512. doi: 10.1016/j.jcin.2019.02.010
19. Watkins S, Good R, Hill J, et al. Intravascular lithotripsy to treat a severely underexpanded coronary stent. EuroIntervention. 2019 May 20; 15(1): 124-125. doi: 10.4244/EIJ-D-18-00780
20. Ali ZA, McEntegart M, Hill JM, Spratt JC. Intravascular lithotripsy for treatment of stent underexpansion secondary to severe coronary calcification. Eur Heart J. 2020 Jan 14; 41(3): 485-486. doi: 10.1093/eurheartj/ehy747.
21. Morabito G, Tripolino C, Tassone EJ, et al. A case of stent under-expansion due to calcified plaque treated with Shockwave lithoplasty. Cardiology. 2018; 141(2): 75-77. doi: 10.1159/000493747
22. Tovar Forero MN, Wilschut J, Van Mieghem NM, Daemen J. Coronary lithoplasty: a novel treatment for stent underexpansion. Eur Heart J. 2019 Jan 7; 40(2): 221. doi: 10.1093/eurheartj/ehy593
23. Chen G, Zrenner B, Pyxaras SA. Combined rotational atherectomy and intravascular lithotripsy for the treatment of severely calcified in-stent neoatherosclerosis: a mini-review. Cardiovasc Revasc Med. 2019 Sep; 20(9): 819-821. doi: 10.1016/j.carrev.2018.10.007
24. Tassone EJ, Tripolino C, Morabito G, Grillo P, Missiroli B. When calcium gets tough, the tough cardiologist starts to play …. Cardiology. 2018;141(3):167-171. doi: 10.1159/000495177
25. Kalogeropoulos AS, Karamasis GV, Pavlidis AN, et al. Combined Shockwave intravascular lithotripsy and ultrahigh-pressure balloon dilatation for the treatment of stent underexpansion secondary to severe coronary calcification. Kardiol Pol. 2021 Feb 25; 79(2): 205-206. doi: 10.33963/KP.15753
26. Alawami M, Thirunavukarasu S, Ahmed J, El-Omar M. Intravascular lithotripsy to treat an underexpanded coronary stent: 4-month angiographic and OCT follow-up. Catheter Cardiovasc Interv. 2020 Nov; 96(6): 1251-1257. doi: 10.1002/ccd.28738
27. Perfetti M, Cocco N, Radico F, et al. Shockwave intravascular lithotripsy for multiple undilatable in-stent restenosis. Cardiol J. 2020; 27(4): 431-432. doi: 10.5603/CJ.2020.0114










