Safe and Effective Calcium Modification in Severe Ostial Right Coronary Artery Stenosis
Editor's note: Don't miss the interview with Srini Potluri, MD at the end of this case. Also, take a quick quiz to test your knowledge of this article here.
CASE
The patient is an 86-year-old male with known coronary artery disease (CAD). Three years ago, he had robotic left internal mammary artery (LIMA) graft to the left anterior descending (LAD) and percutaneous coronary intervention (PCI) of the proximal right coronary artery (RCA). He now has dyspnea on minimal exertion and recent angiography showed a severe, heavily calcified, ostial RCA stenosis. Distal to the stenosis, he had a previous stent that was patent. He was referred to our facility for possible atherectomy and intervention on his ostial RCA.
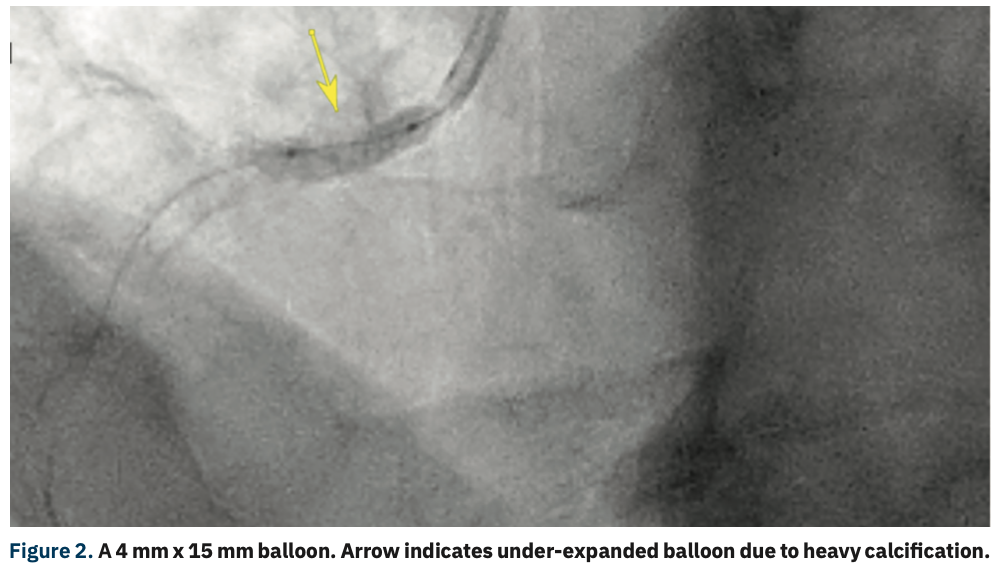
Angiography showed a severe ostial RCA stenosis with thick calcification noted on angiography and intravascular ultrasound (IVUS) showed approximately a 270° arc of calcification (Figure 1). A 4 mm x 15 mm Euphora noncompliant balloon (Medtronic) was initially used to dilate the stenosis, but was under-expanded (Figure 2). At this point, a 4 mm x 12 mm Shockwave C2 coronary intravascular lithotripsy (IVL) balloon (Shockwave Medical) was used and after 2 pulse cycles at 4 atmospheres (atm), the balloon expanded completely (Figure 3). Six more cycles of IVL pulses were performed in the same area. IVUS showed disruption in the calcification. A 4 mm x 16 mm Synergy Megatron stent (Boston Scientific) was deployed at 12 atm and a 4.5 mm Quantum Apex NC balloon (Boston Scientific) was used to post dilate at 20 mm, with good stent expansion and apposition as seen on angiography and IVUS (Figure 4). No complications were encountered and the patient was discharged later that evening in good condition.



Discussion
Several studies have shown the negative impact of coronary calcification on the outcomes of PCI. Angiographically, invisible calcium is common, but doesn’t impact PCI outcomes. However, in moderate to severe angiographic calcification, IVUS and optical coherence tomography (OCT) calcium arc are inversely correlated with stent expansion and outcomes. OCT evaluates the three-dimensional volume of calcific plaque and one can obtain the thickness, length, and circumferential arc of calcium, whereas ultrasound waves reflect off calcium, obviating measurement of calcium thickness.
Coronary IVL was recently approved in the United States. The most striking aspect of using IVL is its ease of use and safety. The serial Disrupt CAD trials evaluated the utility of IVL for lesion preparation of severely calcified coronary stenoses prior to stent implantation. These studies’ primary safety and efficacy endpoints were met among patients from different regions, including the United States, Europe, and Japan. Coronary IVL prior to stent implantation was well tolerated, with a low rate of major periprocedural clinical and angiographic complications and 30-day major adverse cardiac events. OCT imaging provided evidence that calcium fracture was the underlying mechanism of action for coronary IVL.
Numerous algorithms have been developed for calcium management and have been adopted by institutions in several possible ways. We created our own algorithm for calcium management (Figure 5). If, after vessel prep, the imaging doesn’t show disruption or fractures in calcium, then the steps need to be repeated prior to stent deployment. However, if a stent is deployed and noted to be under-expanded, then we would consider IVL with an appropriately sized balloon or laser atherectomy inside the stent to achieve proper stent expansion and apposition. Care must be taken when treating eccentric and nodular calcification. Stent optimization should be performed based on the luminal gain and expansion achieved. Over-inflation may result in expansion into the non-calcified arterial wall and could cause perforation.

Data are limited regarding the use of IVL in ostial coronary lesions. Small series and case reports have been published, showing safety and efficacy. Several devices have been studied for calcium modification, including cutting/scoring balloons, rotational atherectomy (RA), orbital atherectomy (OA), excimer laser atherectomy (ELCA), and now, intravascular lithotripsy. The mechanism by which IVL works includes axial splitting of calcium as the acoustic sound wave travels through the calcific plaque, cavitation and violent collapse of the bubbles in the contrast-saline filled balloon, and fatigue mechanism involving progressive expansion of the micro- and macro-fractures by cumulative repetitive shockwave pulses. Soft tissue is not affected by the Shockwave device.
In comparison with RA and OA, fractures induced by IVL occur along the circumference of the artery, potentially reducing the heterogeneous distribution of resistance to balloon expansion on the arterial wall. Deep dissections were less frequently detected with IVL as compared with 30% after RA and 40% after OA on OCT. To date, no coronary perforations have been observed with IVL in small-size, controlled studies, compared with 0.1-2% with RA and OA. Neither distal embolization nor no reflow have been observed with IVL, compared with an occurrence of up to 2.5% with RA and OA. Due to differences in mechanisms of action, IVL may effectively induce calcium fracture in cases where RA failed to adequately modify calcified lesions. RA, OA, or percutaneous transluminal coronary angioplasty may sometimes be required to facilitate placement of the IVL balloon.4
Conclusion
The presence of moderate to severe coronary calcification impacts procedural success, adverse events, and long-term outcomes. Intravascular imaging is vital in evaluating coronary calcification and management. Stent expansion in heavily calcified lesions may be facilitated by a variety of adjunctive lesion modification technologies that work by different mechanisms of action. IVL is an efficient vessel preparation strategy in the presence of a heavy coronary calcium burden, and is proven to be safe and effective.
Editor's note: Don't miss the interview with Srini Potluri, MD, below. Also, take a quick quiz to test your knowledge of this case and interview with Dr. Potluri.
References
1. Kobayashi Y, Okura H, Kume T, et al. Impact of target lesion coronary calcification on stent expansion. Circ J. 2014; 78(9): 2209-2214. doi: 10.1253/circj.cj-14-0108
2. Ali ZA, Karimi Galougahi K, Maehara A, et al. Intracoronary optical coherence tomography 2018: current status and future directions. JACC Cardiovasc Interv. 2017 Dec 26; 10(24): 2473-2487. doi: 10.1016/j.jcin.2017.09.042
3. Kereiakes DJ, Di Mario C, Riley RF, et al. Intravascular lithotripsy for treatment of calcified coronary lesions: patient-level pooled analysis of the Disrupt CAD studies. JACC Cardiovasc Interv. 2021 Jun 28; 14(12): 1337-1348. doi: 10.1016/j.jcin.2021.04.015
4. Karimi Galougahi K, Shlofmitz E, Jeremias A, et al. Therapeutic approach to calcified coronary lesions: disruptive technologies. Curr Cardiol Rep. 2021 Mar 5; 23(4): 33. doi: 10.1007/s11886-021-01458-7
5. Yarusi BB, Jagadeesan VS, Jivan A, et al. The utility of peripheral intravascular lithotripsy in calcific coronary artery disease: a case series. J Invasive Cardiol. 2021 Apr; 33(4): E245-E251.
6. Karimi Galougahi K, Patel S, Shlofmitz RA, et al. Calcific plaque modification by acoustic shock waves: intravascular lithotripsy in coronary interventions. Circ Cardiovasc Interv. 2021 Jan; 14(1): e009354. doi: 10.1161/CIRCINTERVENTIONS.120.009354
––––––––––––––––––
Interview
Q&A With the Operator

Can you tell us about your work and your experience with intravascular lithotripsy?
Baylor Scott and White The Heart Hospital Plano is a specialty hospital that does approximately 2000 percutaneous coronary interventions (PCIs) per year, mostly referrals by other interventional cardiologists in the area. We have several cath labs as well as several hybrid rooms in our hospital. I am an interventional cardiologist, I direct the cath lab at the Heart Hospital, and I am the chair of cardiology.
I have been using intravascular lithotripsy (IVL), which I believe is an important technology, since its inception. Peripheral IVL use has been cleared for some time and coronary use has recently been approved. We have been using IVL in peripheral intervention for quite a long time. Right now, one of our biggest uses for IVL is using the peripheral device in patients with severe calcific iliac arteries prior to transcatheter aortic valve replacement (TAVR). It has allowed us to avoid using alternative access in a significant number of patients.
Were you able to take any knowledge from your peripheral experience with IVL for coronary application of the device?
Yes. The basic appeal of IVL is its safety, and we brought that awareness of the safety of the technology to our coronary applications. We confirmed in the Disrupt CAD trial that IVL is safe. We feel comfortable using it in patients who have significant calcific coronary artery disease.
The advantage of the Shockwave device is that we inflate the balloon to such a low atmospheric pressure: 4 atmospheres (atm). If you look at other technologies available for coronary calcific stenosis, whether rotational atherectomy, cutting balloons, or orbital atherectomy, all of these devices have safety issues, and a high risk of morbidity and major adverse cardiac events. IVL allows you to use very low inflation pressures and fracture the lesion with lithotripsy energy, allowing for better luminal gain.
How does IVL fit into your treatment algorithm?
Our algorithm for IVL (Figure 5) involves the failure of a 1:1 noncompliant balloon. If I put in a noncompliant balloon, inflate it at 12 atmospheres, and still have a waist, then I pull out an IVL balloon. We have found that in these cases, IVL usually works to fracture the calcium and prepare the vessel for stenting.
Is concentric calcium the best-case scenario for IVL?
Yes. It is fairly random as to whether someone has concentric versus nodular calcium. We may see both in the same patient. IVL can be used to help with expansion of the concentric calcium.
How are you assessing coronary calcium?
Mostly on the angiogram. If we have computed tomography angiography (CTA), we look at the CTA as well.
Are you using intravascular ultrasound (IVUS)?
We do use a fair amount of IVUS, and even with IVUS, sometimes it is hard to tell if the lesion will expand. IVUS does not permit you to evaluate the thickness of the calcium. For most of these patients with heavy calcium, and especially calcium with some tortuosity or severe disease, I can’t even get my IVUS catheter down. I think the best way to see if the lesion will expand or the calcium will crack is by using a noncompliant balloon. The other issue is that if a patient has a severe stenosis and I am not able to get anything through, then I still have to consider using rotational atherectomy before I can get any balloon through. Sometimes with rotational atherectomy, although we score the calcium, expansion is still difficult, unless you do significant balloon angioplasty on the calcific lesion. There are multiple situations where I have to use rotational atherectomy and IVL to get better expansion of the lesion and the stent.
Does the safety of IVL lend itself to use in more tortuous areas?
Definitely. If there is a tortuous coronary with calcium that will not expand with a noncompliant balloon, I prefer IVL versus rotational atherectomy. Also, if I can get a balloon across, then I know that using IVL is going to take less time than setting up and using rotational atherectomy. The biggest question is whether I am able to deliver the IVL device. Definitely, however, IVL has an advantage over rotational atherectomy in tortuous lesions, including bifurcations. With bifurcation disease, IVL can be used in both branches without losing the wire position. If I have to use rotational atherectomy, I have to take the wire out of the side branch.
When you use the Shockwave device, do you use all the 80 pulses or do you hold any in reserve?
If I open a balloon, I try to use all of the 80 seconds of time. However, if I go in and on the first try, the lesion is now wide open, I may not use all of it. If I am treating two branches with IVL, I am going to hit both the branches separately, but I try to maximize how much I use, meaning treating both branches with one Shockwave balloon. If one branch is 2.5 mm and the other branch is 4 mm, then I may use a separate balloon. But if both branches are comparably or relatively the same diameter, then I probably will use the same balloon for both branches.
Has the pass-through payment helped with IVL use at your lab?
One of the biggest initial factors was the cost of the therapy. Now, with the added code, we are seeing more and more usage of IVL in multiple cases. In the beginning, when IVL first came out, I created a protocol as to when IVL could be pulled by our staff. But now, anyone can utilize IVL if the lesion failed a noncompliant balloon. With that in mind, we do perform a high volume of complex calcific coronary artery disease procedures, particularly chronic total occlusion (CTO) interventions. If you are subintimal through the calcium, you cannot get good results without appropriate balloon expansion of CTO segments. IVL does help significantly by allowing us to get good stent apposition and open that lumen. In complex calcific coronary artery disease, there is definitely a safety margin where we tend to pull IVL sooner than later.
Any final thoughts?
We do know that both stent apposition and proper stent sizing matter. We try to get the patient out of the hospital with the best possible outcome. IVL is the way to go, especially in terms of safety.
Editor's note: Take a quick quiz to test your knowledge of this article here.
This article is sponsored by Shockwave Medical.
Dr. Srini Potluri is a paid consultant for Shockwave Medical. See Important Safety Information below.
Learn more about coronary intravascular lithotripsy use by visiting Cath Lab Digest’s Calcium Corner.
CLD home page –> Topics –> Calcium Corner
Visit Calcium Master Class

Visit Calcium Master Class
Rx only
Indications for Use— The Shockwave Intravascular Lithotripsy (IVL) System with the Shockwave C2 Coronary IVL Catheter is indicated for lithotripsy-enabled, low-pressure balloon dilatation of severely calcified, stenotic de novo coronary arteries prior to stenting
Contraindications— The Shockwave C2 Coronary IVL System is contraindicated for the following: This device is not intended for stent delivery. This device is not intended for use in carotid or cerebrovascular arteries.
Warnings— Use the IVL Generator in accordance with recommended settings as stated in the Operator’s Manual. The risk of a dissection or perforation is increased in severely calcified lesions undergoing percutaneous treatment, including IVL. Appropriate provisional interventions should be readily available. Balloon loss of pressure was associated with a numerical increase in dissection which was not statistically significant and was not associated with MACE. Analysis indicates calcium length is a predictor of dissection and balloon loss of pressure. IVL generates mechanical pulses which may cause atrial or ventricular capture in bradycardic patients. In patients with implantable pacemakers and defibrillators, the asynchronous capture may interact with the sensing capabilities. Monitoring of the electrocardiographic rhythm and continuous arterial pressure during IVL treatment is required. In the event of clinically significant hemodynamic effects, temporarily cease delivery of IVL therapy.
Precautions— Only to be used by physicians trained in angiography and intravascular coronary procedures. Use only the recommended balloon inflation medium. Hydrophilic coating to be wet only with normal saline or water and care must be taken with sharp objects to avoid damage to the hydrophilic coating. Appropriate anticoagulant therapy should be administered by the physician. Precaution should be taken when treating patients with previous stenting within 5mm of target lesion.
Potential adverse effects consistent with standard based cardiac interventions include– Abrupt vessel closure - Allergic reaction to contrast medium, anticoagulant and/or antithrombotic therapy-Aneurysm-Arrhythmia-Arteriovenous fistula-Bleeding complications-Cardiac tamponade or pericardial effusion-Cardiopulmonary arrest-Cerebrovascular accident (CVA)-Coronary artery/vessel occlusion, perforation, rupture or dissection-Coronary artery spasm-Death-Emboli (air, tissue, thrombus or atherosclerotic emboli)-Emergency or non-emergency coronary artery bypass surgery-Emergency or non-emergency percutaneous coronary intervention-Entry site complications-Fracture of the guide wire or failure/malfunction of any component of the device that may or may not lead to device embolism, dissection, serious injury or surgical intervention-Hematoma at the vascular access site(s)-Hemorrhage-Hypertension/Hypotension-Infection/sepsis/fever-Myocardial Infarction-Myocardial Ischemia or unstable angina-Pain-Peripheral Ischemia-Pseudoaneurysm-Renal failure/insufficiency-Restenosis of the treated coronary artery leading to revascularization-Shock/pulmonary edema-Slow flow, no reflow, or abrupt closure of coronary artery-Stroke-Thrombus-Vessel closure, abrupt-Vessel injury requiring surgical repair-Vessel dissection, perforation, rupture, or spasm.
Risks identified as related to the device and its use: Allergic/immunologic reaction to the catheter material(s) or coating-Device malfunction, failure, or balloon loss of pressure leading to device embolism, dissection, serious injury or surgical intervention-Atrial or ventricular extrasystole-Atrial or ventricular capture.
Prior to use, please reference the Instructions for Use for more information on warnings, precautions and adverse events. www.shockwavemedical.com/IFU
Please contact your local Shockwave representative for specific country availability and refer to the Shockwave C2 Coronary IVL system instructions for use containing important safety information.
©2021 Shockwave Medical Inc., All rights reserved. SPL 65758 Rev.A










