What’s Your Hemodynamic IQ? Some Questions and Answers Regarding Hemodynamic Calculations and Other Issues in the Cath Lab
 I love hemodynamics in the cath lab and appreciate the opportunity to address problems with measurements and their interpretation. Dr. Safwan Kassas, from the Michigan Cardiovascular Institute, asked the following questions concerning hemodynamic calculations.
I love hemodynamics in the cath lab and appreciate the opportunity to address problems with measurements and their interpretation. Dr. Safwan Kassas, from the Michigan Cardiovascular Institute, asked the following questions concerning hemodynamic calculations.
1. A high cardiac output during right heart catheterization (RHC) in the absence of sepsis should trigger suspicion for intra-cardiac shunting. What cardiac output (CO) values should raise my suspicion and start my diagnostic work-up for shunting?
Normal PA O2 saturations range from 65-75%. Values higher than 75% indicate a higher than usual cardiac output, that may or may not be appropriate for the clinical presentation. The absolute value of the cardiac output is not what triggers the investigation of a shunt, but rather the increase in O2 saturation between right heart chambers. When doing a right heart catheterization, one should always obtain a superior vena cava (SVC) or inferior vena cava (IVC) saturation and pulmonary artery (PA) saturation, at the minimum. If the O2 step-up (increase) between chambers (IVC/SVC vs. PA) exceeds 7%, then the possibility of left-to-right shunt must be excluded. For right-to-left shunts, this is not usually a silent entity and one should have clinical findings, meaning increased right atrial, right ventricular, and PA pressures, to have high suspicion for right-to-left shunts.
2. Does sinus tachycardia or sinus bradycardia affect the pressure gradient across the aortic valve?
Pressure gradients change with increasing or decreasing flow. Normal valves have no gradient and changes in heart rate will not artificially produce a gradient. In valves with stenoses, sinus tachycardia usually produces increased cardiac output (stroke volume x heart rate), and thus the increased flow will increase an aortic or mitral valve gradient. Bradycardia produces longer periods of diastole and will not often affect aortic gradients unless associated with lower cardiac output, but may reduce mitral gradients, because of the longer time permitting decompression of the left atrial (LA) flow into the left ventricle (LV).
3. When calculating CO using the thermodilution method, what is the correct volume to inject, 5cc or 10cc? Does the speed of injection matter?
Thermodilution cardiac output accuracy depends on several factors. Injection of bolus saline at room temperature is as good as cold saline. The bolus injected through the right atrial port should be quick, so as not to let the bolus spread through dilution. The quick bolus must mix completely, with blood traveling through the RV into the PA, where it is detected and the area under the temperature curve computed to produce the cardiac output number. Interference with the bolus transit after injection by tricuspid regurgitation or stenosis, shunts, or very low cardiac output will produce a wide variation of measurements. It is customary to inject 10cc of saline bolus. Remember to input the right calibration factor on the thermodilution cardiac output computer (TD instrument) for the catheter being used.
4. Should we not use the thermodilution method to calculate CO if there is more than moderate tricuspid regurgitation? What are the other circumstances where the thermodilution method should not be used to calculate CO?
As noted above, tricuspid regurgitation or stenosis, shunts, or very low cardiac output will produce unreliable CO results. In these circumstances (in fact, with all right heart caths as a double check), use the PA-arterial O2 saturations and compute a FICK cardiac output.
Cardiac output (CO) using the Fick principle (O2 consumption) is calculated as follows:
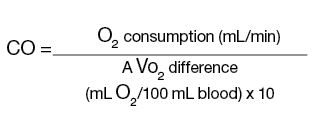
Oxygen consumption is estimated as 3ml O2/kg or 125ml/min/m2. Arteriovenous oxygen (AVO2) difference is calculated from arterial - mixed venous (PA) O2 content, where O2 content = saturation x 1.36 x hemoglobin. For example, if the arterial saturation is 95%, then the O2 content = 0.95 x 1.36 x 13.0 g = 16.7 ml, PA saturation is 65%, and O2 consumption is 210ml/min (70 kg x 3ml/kg) or measured value, and CO would be determined as follows:
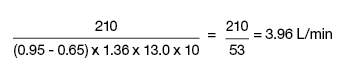
5. In the HAKKI equation, do we use the peak gradient or the mean gradient (I have read both)?
The HAKKI equation is the simplified formula for computing valve areas. Aortic valve area can be accurately estimated as CO divided by the square root of the LV-aortic peak-to-peak pressure difference. For the mitral valve area, it uses the mean LA/pulmonary capillary wedge (PCW) to mean LV diastolic pressure, values much harder to ‘eyeball’ with accuracy. For example, in a patient with aortic stenosis, if the peak-to-peak gradient = 65 mm Hg and the CO = 5 L/min, then:
Quick valve area (Hake formula) =
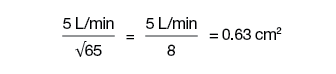 Recall that the quick formula for valve area differs from the Gorlin formula by 18 ± 13% in patients with bradycardia (<65 beats/min) or tachycardia (>100 beats/min). The Gorlin equation at low-flow states overestimates the severity of valve stenosis.
Recall that the quick formula for valve area differs from the Gorlin formula by 18 ± 13% in patients with bradycardia (<65 beats/min) or tachycardia (>100 beats/min). The Gorlin equation at low-flow states overestimates the severity of valve stenosis.
6. Low ejection fraction or severe stenotic valves lead to reduced CO. Are there other scenarios that can lead to underestimation of the true CO?
Of course, the measurement of accurate CO depends on the technique and clinical scenarios. For TD cardiac output, the main confounders were discussed above. For the FICK cardiac output, accurate measurement of O2 saturations and the correct computation of O2 consumption are the sources of error. Low outputs are associated with high O2 extraction, as the blood travels around the body such that the returning O2 saturation to the right heart will be low, usually lower than 65%, and often 55%. When PAO2 saturation is 40%, cardiac output is usually <1.5L/m, and the patient is critically ill, near death.
7. When measuring fractional flow reserve (FFR) of the ostial left main or ostial right coronary artery (RCA), I believe adenosine should be given IV with the guide catheter not engaged. Is it better to give adenosine as rapid IV boluses or as an IV drip? If given as a drip, how much time is needed before I make the FFR measurement? What is the concentration of adenosine IV?
I recommend using IV infusion of adenosine at constant rate for all FFR measurements. IV adenosine is required to measure ostial lesions, because the guide catheter may obstruct ostial flow and is required to measure pressure pull back on a continuous basis. Infuse 140mcg/kg/min through a large vein (not a hand vein; the bigger vein, the better), wait to see the arterial pressure drop and the heart rate increase about 2 minutes into the infusion, and measure the lowest value of FFR. In some patients, with IV given in a small hand or forearm vein, there may be fluctuations in the adenosine concentration as venous return may vary and hence FFR can be low, then rise, as the adenosine hyperemia is blunted from lower venous return. Take the lowest FFR in this case.
8. When evaluating for reversibility of pulmonary hypertension, is a 20% reduction in pulmonary vascular resistance (PVR) with adenosine infusion in the PA catheter sufficient to prove reversibility? If I use escalating doses of adenosine, I do need to measure the new CO after each dose to calculate the corresponding PVR for that adenosine dose?
The standard for assessing pulmonary vasoreactivity is to measure pressures and O2 saturations after inhaling 20ppm of nitric oxide for 5 minutes. Measurements are obtained before, at peak (4 minutes) and after washout of NO (2 min more). A 20% reduction in PVR is good, but we look at absolute PA pressure reduction as well. I am not familiar with adenosine being used in this fashion and it is not recommended for this approach with any substantial supporting data.
9. After using sequential balloon inflations for balloon valvuloplasty, in order to calculate the final aortic valve area (AVA), I will need to re-measure the CO after the final balloon inflation and not rely on the original CO, is that correct? If my valvuloplasty procedure ends up with moderate to severe aortic insufficiency, how would that affect my pressure gradient, thermodilution CO, and AVA calculations in the cath lab?
Final AVA post balloon inflation is calculated from the remaining LV-aortic (Ao) pressure gradient and the cardiac output. For best accuracy, use the CO at the time of final gradient measurement. CO might change due to induced ischemia, LV dysfunction, or tachycardia. Regarding how aortic insufficiency affects hemodynamics, the regurgitant valve permits blood to leak back into the LV with the potential to reduce forward flow, but this is usually compensated for by greater stroke volume. You can use either FICK or TD outputs. The LV-Ao gradient will reflect the valve area accurately if CO is accurately measured.
 Bonus Question #1, from Arthur Tjin, International Clinical and Technical Training Manager,
Bonus Question #1, from Arthur Tjin, International Clinical and Technical Training Manager,
ACIST Corporation:
I always teach people that the physical location of the pressure transducer is of no importance provided the level of the transducer doesn’t change during the procedure. The zero-reference is obtained from the stockcock connecting pressure tubing with the catheter. Zero at the beginning of the case is set at the mid axillary level.
We face ‘old school’ mentality of CCL personnel worldwide who do not wish to believe that you can move the connecting tube to zero without having to move the transducer. Can you clarify this problem for me?
In our lab, the transducer at the side of the bed is connected to tubing and filled with saline. The end of the tubing has a stopcock that we open to air to register the atmospheric pressure (zero) at the place we put that tubing (mid chest, on top of the chest, at the leg, whereever we estimated mid-RA position is). If you raise the tubing up, the registered pressure will go down and vice versa (see Figure 1). Once the zero point is set, do not move the transducer. It does not matter at what height the transducer is to start with.
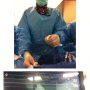
I put a 5 French (F) balloon tipped pacemaker catheter into an 8F SG sheath and it leaks. Do I have to use a bigger pacer catheter to stop the leak?
No, use a 6F sheath in the 8F sheath, then put the 5F pacer through the 6F sheath (see Figure 2). Voila, no leak.
Reference
- Kern MJ, ed. The Cardiac Catheterization Handbook. 5th ed. Philadelphia: Elsevier; 2011.
- Kern MJ, Lim MJ, Goldstein JA, eds. Hemodynamic Rounds: Interpretation of cardiac pathophysiology from pressure waveform analysis. 3rd ed. New York: Wiley-Liss; 2009.
__________________________________________
LETTER TO THE CLINICAL EDITOR
Re: “Retool or Retire” (from the March 2013 Editor’s Corner)
Dr. Kern,
As a 66 y.o. physician, I enjoyed your article about keeping up with change. However, I disagree with you about bivalirudin and prasugrel in non-MI patients, based on ISAR-REACT 3, which did not show net clinical benefit for bivalirudin. Bleeding is not an issue with the radial approach. My 2013 approach is radial with heparin and prasugrel or ticagrelor loading after anatomy is identified. (Bivalrudin is very expensive.) If they are already on clopidogrel and an elective case, I continue this, as I know of no data that the new agents are better and they do not have the indication.
Alan Kudler, The Hospital of Central Connecticut, New Britain, CT
Dr. Kudler reports no conflicts of interest.
Dear Dr. Kudler,
Thank you for your letter about “Retool or Retire.” I see you have retooled and adopted the radial-first approach. My compliments. With regard to bivalirudin and prasugrel, I don’t believe this was meant as a recommendation, but rather an illustration about considering, then adopting, improved approaches. In the preceding paragraph to the table, it is mentioned that if a new approach fails the test of time, one has to reconsider and move on. If two approaches are equal regarding benefits, it makes sense to keep the less expensive. But, if not, then the fact that something is more expensive should not be a factor if the benefit over the prior methods/approaches is substantial. Witness DES over BMS.
Best regards,
Morton
Kern