Ventricular Septal Defect in an Acute Myocardial Infarction
This case received double-blind peer review from members of the Cath Lab Digest editorial board.
Disclosures: The authors report no conflicts of interest regarding the content herein.
The authors can be contacted via Raymond Lenius, MS, RCIS, at raymond.lenius@fsw.edu.
Introduction
A ventricular septal defect (VSD) is an abnormal communication between the left and right ventricle through a defect in the septal wall. In most cases, this will cause oxygenated blood to flow from the left ventricle into the right ventricle and mix with deoxygenated blood. If a VSD results from an acute myocardial infarction (AMI), it is also referred to as a ventricular septal rupture (VSR). A VSR is uncommon and occurs only 1-2% of the time.1
The other type of VSD is congenital, which accounts for more than 20% of all congenital heart diseases.2 We report a case of a 62-year-old male that developed a VSR after having an AMI. The patient presented to the emergency department (ED) from a local outpatient clinic following 4.5 hours of chest pain.
Case presentation
A 62 year-old male was awakened by a sudden onset of chest pain and some indigestion at 4:00 am. The patient decided to go to a local outpatient clinic instead of the local ED. Upon his arrival at the clinic, the staff did an electrocardiogram (ECG), and after seeing it was abnormal, the patient was sent emergently to the local ED. The patient arrived at the ED approximately 4.5 hours following the onset of chest pain. Upon arrival, another ECG was performed (Figure 1) and it was determined the patient was having an anterolateral ST elevation myocardial infarction (STEMI), and that he had suffered from an inferior wall MI in the past. At this time, a cardiology consult was requested and following the exam, the patient was brought to the catheterization lab for an emergency heart catheterization.
The patient’s history is as follows:
- No known drug allergies
- Former smoker
- Mild alcohol consumption
- No drug abuse
- Hypertension
- Tenormin 25mg 1x/daily
- No diabetes
- Asthma
- Peptic ulcer disease
- No family history of heart disease
The examination by the cardiologist revealed:
- Alert and oriented x 3, no fever
- Chest pain (2 out of 10)
- No palpations
- No shortness of breath or cough
- No neurological problems
- Mild gastrointestinal discomfort
- No jugular vein distention
- No edema
- A harsh systolic ejection murmur
- No S3 or S4 sounds
His vitals and labs were:
- Blood pressure (BP) 115/80
- Pulse 99
- Respirations 22
- White blood cell count (WBC) 14
- Hemoglobin 16
- Hematocrit 48
- Glucose 137
- Platelets 188
- Blood urea nitrogen (BUN) 24
- Creatinine 1.4
- Creatine phosphokinase (CPK) 542
- Troponin 6.2
Procedure
After a complete examination, the patient was brought to the cardiac catheterization lab for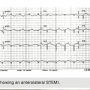 emergency cardiac catheterization. When the patient arrived at the catheterization lab, nitroglycerin (NTG) was being infused at 10 mcg/min and the patient was on oxygen via nasal canula at 2 liters per minute (LPM). The patient was placed on the table, and was prepped and draped in the usual sterile fashion. After local anesthetic was administered to the right groin, the right femoral artery was accessed using the modified Seldinger technique. A 6 French (Fr) sheath (Terumo) was advanced over the wire into the right femoral artery. A 6 Fr femoral left (FL) 4 diagnostic catheter (Boston Scientific) was advanced over a 0.035-inch guide wire. Opening aortic pressure was 105/76. The FL4 catheter was unable to cannulate the left coronary artery and was removed over the guide wire. Next, a 6 Fr FL5 diagnostic catheter (Boston Scientific) was advanced over the guide wire. The left coronary artery was cannulated and contrast was injected to visualize the artery. Angiograms revealed a 100% occlusion in the left anterior descending (LAD) artery just distal to the first diagonal branch. The first diagonal
emergency cardiac catheterization. When the patient arrived at the catheterization lab, nitroglycerin (NTG) was being infused at 10 mcg/min and the patient was on oxygen via nasal canula at 2 liters per minute (LPM). The patient was placed on the table, and was prepped and draped in the usual sterile fashion. After local anesthetic was administered to the right groin, the right femoral artery was accessed using the modified Seldinger technique. A 6 French (Fr) sheath (Terumo) was advanced over the wire into the right femoral artery. A 6 Fr femoral left (FL) 4 diagnostic catheter (Boston Scientific) was advanced over a 0.035-inch guide wire. Opening aortic pressure was 105/76. The FL4 catheter was unable to cannulate the left coronary artery and was removed over the guide wire. Next, a 6 Fr FL5 diagnostic catheter (Boston Scientific) was advanced over the guide wire. The left coronary artery was cannulated and contrast was injected to visualize the artery. Angiograms revealed a 100% occlusion in the left anterior descending (LAD) artery just distal to the first diagonal branch. The first diagonal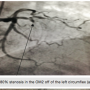 branch had a 60 to 70% stenosis at its ostium. The second obtuse marginal off the left circumflex artery revealed an 80% stenosis (Figure 2). Next, a 6 Fr femoral right (FR) 4 diagnostic catheter (Boston Scientific) catheter was advanced over the guide wire. The right coronary artery (RCA) was cannulated and contrast was injected to visualize the artery. Angiograms revealed a 50% stenosis in the mid portion (Figure 3). The posterior descending artery (PDA) was free of disease.
branch had a 60 to 70% stenosis at its ostium. The second obtuse marginal off the left circumflex artery revealed an 80% stenosis (Figure 2). Next, a 6 Fr femoral right (FR) 4 diagnostic catheter (Boston Scientific) catheter was advanced over the guide wire. The right coronary artery (RCA) was cannulated and contrast was injected to visualize the artery. Angiograms revealed a 50% stenosis in the mid portion (Figure 3). The posterior descending artery (PDA) was free of disease.
Intervention was performed on the occlusion in the LAD. Bivalirudin was given as an anticoagulant prior to the insertion of the guide catheter. A 6 Fr CLS 4.0 guide catheter (Boston Scientific) was advanced over the guide wire and the LCA was cannulated. An Asahi Prowater 0.014-inch wire (Abbott Vascular) was advanced into the distal LAD past the occlusion. An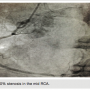 Export aspiration catheter (Medtronic) was advanced into the LAD over the Prowater wire. Aspiration of the LAD was performed, and thrombus was removed. An angiogram was taken of the LAD following the aspiration, which revealed the LAD was patent, but there was a residual dissection at the site of the occlusion. A 3.0 x 16 mm Promus Element drug-eluting stent (Boston Scientific) was advanced over the Prowater wire and placed at the dissection, and the stent was deployed at 13 atmospheres. After the stent was deployed, a good result was demonstrated by angiogram (Figure 4). The stent deployment system and guide wire were removed, and the final angiograms were taken. The post interventional angiograms showed that the culprit lesion in the LAD was reduced to 0%, and there was TIMI-III flow to the distal vessel.
Export aspiration catheter (Medtronic) was advanced into the LAD over the Prowater wire. Aspiration of the LAD was performed, and thrombus was removed. An angiogram was taken of the LAD following the aspiration, which revealed the LAD was patent, but there was a residual dissection at the site of the occlusion. A 3.0 x 16 mm Promus Element drug-eluting stent (Boston Scientific) was advanced over the Prowater wire and placed at the dissection, and the stent was deployed at 13 atmospheres. After the stent was deployed, a good result was demonstrated by angiogram (Figure 4). The stent deployment system and guide wire were removed, and the final angiograms were taken. The post interventional angiograms showed that the culprit lesion in the LAD was reduced to 0%, and there was TIMI-III flow to the distal vessel.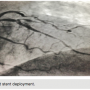 The patient denied any pain after the deployment of the stent. The guide catheter was removed, and the intravenous nitroglycerin was discontinued.
The patient denied any pain after the deployment of the stent. The guide catheter was removed, and the intravenous nitroglycerin was discontinued.
A 6 Fr angled pigtail diagnostic catheter (Boston Scientific) was advanced over the guide wire and placed in the left ventricle (LV). The pressure in the LV was 101/2 with an end diastolic pressure (EDP) of 16 mmHg. An LV gram was performed in the right anterior oblique (RAO) projection using a power injector. The settings used for the LV gram were 15ml/sec for a total of 35ml. During the LV gram, it was noticed that the right ventricle (RV) was also filling (Figure 5). When the cardiologist observed this, he suspected the patient might have a VSR. Following the LV gram, the pigtail was pulled back across the aortic valve and no gradient was observed. The bivalirudin infusion was discontinued at this time.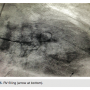
In order to confirm and quantify the VSR, the physician decided to proceed with a right heart catheterization. Additional anesthetic was injected into the right groin, and the right femoral vein (RFV) was accessed using the modified Seldinger technique. A 7 Fr sheath (Terumo) was advanced over the wire into the RFV. A 7 Fr Swan Ganz Thermodilution (Swan) catheter (Vascular Solutions) was advanced into the RFV and advanced. The Swan was placed in the following areas, where pressures and/or blood samples were drawn:
- Inferior vena cava (IVC) – SvO2 = 51%, no pressure measured;
- Right atrium (RA) – SvO2 = 45%, no pressure measured;
- Right ventricle – SvO2 = 91%, Pressure = 58/9, RVEDP = 22;
- Pulmonary artery (PA) – SvO2 = 81%, Pressure = 50/23/36;
- Pulmonary capillary wedge (PCW) – Pressure = 25/40/28;
- The SaO2 in the RFA was 95%.
The VSR was confirmed by the step-up in saturations between the RA and RV. The cardiothoracic surgeon was called at this time to discuss emergency surgery to repair the VSR. It was decided that an intra-aortic balloon pump (IABP) would be inserted. The 6 Fr sheath in the RFA was removed and the 8 Fr sheath from the intra aortic balloon (IAB) was advanced. Proper IAB placement was confirmed by fluoroscopy. The IAB sheath and IAB was sutured in place, as well as the Swan. The settings on the IABP were 1:2, augmented diastolic BP 113 mmHg, and the ECG was used as the trigger. After the IABP was in place, the following medications were given:
- NTG drip restarted at 10 mcg/min;
- Oxygen increased to 3 LPM nasal canula;
- Lasix 20 mg IV;
- Heparin drip was started at 12 units/kg/hr;
- Integrilin bolus of 17 mg was given IV.
Following the catheterization, an echocardiogram was performed. The echocardiogram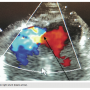
- Ejection fraction 35 to 40%;
- LV apical dyskinesis;
- Apical VSR;
- Left to right ventricular shunt.
The cardiologist knew that the VSR needed to be repaired to increase the likelihood of survival for the patient. Therefore, after speaking with the surgeon, it decided the patient would be transferred to another facility so the VSR could be surgically repaired. Upon arrival at the receiving hospital, the patient was brought to the operating room and was prepped for surgery. During the surgery, the posterior descending artery (PDA) and the obtuse marginal (OM) artery were bypassed, and the VSR repaired. After the surgeon accessed the infarcted area of the LV and RV, he was able to visualize that the VSR was located in the apex of the ventricles and measured about 3 cm. The repair of the rupture was done using a synthetic graft material called CorMatrix. After repairing the VSR, the patient was weaned from bypass, and at this time, the LV began to rupture towards the lateral wall. The OR team tried to stop the rupture, but despite their best efforts, the patient did not survive and expired in the operating room.
transferred to another facility so the VSR could be surgically repaired. Upon arrival at the receiving hospital, the patient was brought to the operating room and was prepped for surgery. During the surgery, the posterior descending artery (PDA) and the obtuse marginal (OM) artery were bypassed, and the VSR repaired. After the surgeon accessed the infarcted area of the LV and RV, he was able to visualize that the VSR was located in the apex of the ventricles and measured about 3 cm. The repair of the rupture was done using a synthetic graft material called CorMatrix. After repairing the VSR, the patient was weaned from bypass, and at this time, the LV began to rupture towards the lateral wall. The OR team tried to stop the rupture, but despite their best efforts, the patient did not survive and expired in the operating room.
Discussion
Three mechanical complications from an AMI are ventricular free wall rupture (VFWR), ventricular septal rupture (VSR), and papillary muscle rupture with severe mitral regurgitation (MR). While the VFWR and VSR are uncommon, they carry a high mortality rate.3 The risk factors for septal rupture include advanced age (>65 years), female gender, single-vessel disease (mostly from an occluded LAD), extensive MI, and poor septal collateral circulation.4 If a patient is treated medically, the mortality rate is 85% within 2 months.5
Ventricular septal rupture can be life threatening, and has a high mortality rate. According to the guidelines from the American College of Cardiology/American Heart Association, the best treatment for a VSR is urgent surgical intervention, regardless of the patient’s clinical status.4 In order to help prevent death prior to surgery, and increase the chances of the surgery being successful, physicians may consider putting in an IABP, give oxygen, start a vasodilator, administer diuretics, and give an inotropic agent.4 There is no data about the risks or benefits of using positive inotropic agents.6 Therefore, positive inotropic agents should only be used in patients that are hypotensive (systolic BP < 90 or 80 mmHg) or are in cardiogenic shock.6 In this case, following these guidelines prevented the patient from going into cardiogenic shock. According to the SHOCK trial (Should We Emergently Revascularize Occluded Coronaries for Cardiogenic Shock), the mortality rate for a patient with a VSR that develops cardiogenic shock is higher.4 Patients with VSR should only be treated medically until surgery can be performed. According to the GUSTO-I trial (Global Utilization of Streptokinase and TPA for Occluded Coronary Arteries), the 30-day mortality rate was lower in patients with a VSR who underwent surgical repair versus patients treated medically (47% vs. 94%), as was the 1-year mortality rate (53% vs. 97%, respectively).4
At the end of the catheterization lab procedure, the patient was pain free and in stable condition. In this case, the physician followed the guidelines set forth by the American College of Cardiology/American Heart Association for VSRs. By following these guidelines, the physician was able to keep the patient in stable condition; thus giving the patient the best possible chance for survival. However, even this was not enough to overcome the damage done to the septal wall. This is an excellent example of why it is important for anyone suspecting a myocardial infarction to seek immediate medical attention. In addition, it is important for every member of the catheterization team to be vigilant and aware of potential complications, even if the likelihood of complications may be low.
References
- Bhimji S. Postinfarction ventricular septal rupture. Available online at https://emedicine.medscape.com/article/428240-overview. Updated February 11, 2013. Accessed June 17, 2014.
- Ramaswamy P, Srinivasan K. Ventricular septal defects. Available online at https://emedicine.medscape.com/article/892980-overview. Updated April 17, 2013. Accessed June 17, 2014.
- Yip HK, Wu CJ, Chang HW, Wang CP, Cheng CI, Chua S, Chen MC. Cardiac rupture complicating acute myocardial infarction in the direct percutaneous coronary intervention reperfusion era. Chest. 2003 Aug; 124(2): 565-571.
- Kondur AK, Hari P, Afonso LC. Complications of myocardial infarction. Available online at https://emedicine.medscape.com/article/164924-overview. Updated May 10, 2013. Accessed June 17, 2014.
- Coskun KO, Coskun ST, Popov AF, Hinz J, Schmitto JD, Bockhorst K, Stich KM, Koerfer R. Experiences with surgical treatment of ventricle septal defect as a post infarction complication. J Cardiothorac Surg. 2009 Jan 6; 4: 3. doi: 10.1186/1749-8090-4-3.
- Gul I, Zorlu A, Yılmaz M, Karapınar H, Küçükdurmaz Z. The effects of levosimendan in a patient with postinfarction ventricular septal defect. Open Access Scientific Reports 2012; 1(8): 415. doi: 10.4172/scientificreports.415.










