Transradial Revascularization of a Chronic Total Subclavian Occlusion
 Case report
Case report
A 65-year-old male presented with the chief complaint of angina on exertion for about 8 to 9 months. He had retrosternal chest discomfort, along with left arm heaviness when he would paint walls, and he could not continue to work as a painter for about a month due to worsening of his symptoms. His past medical history is significant for a coronary artery bypass surgery six years ago with left internal mammary artery (LIMA) to left anterior descending coronary artery (LAD), saphenous vein graft (SVG) to posterior descending artery (PDA) and SVG to obtuse marginal (OM). He also had a history of hypertension, active tobacco use and dyslipidemia. On physical examination, he had a weak left radial pulse in comparison to the right, along with a 38 mm lower blood pressure in the left arm in comparison to the right arm. The rest of the physical exam was unremarkable. With high clinical suspicions for coronary artery disease (CAD) and left subclavian artery stenosis, the patient was sent to the cardiac cath lab for further evaluation and management.
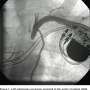 A 6 French (Fr) Glidesheath (Terumo) was inserted in the left radial artery. A Judkins right (JR) 4 catheter was advanced from the left radial over a J wire. The left subclavian angiogram showed a 100% chronic total occlusion (CTO) of the left SA proximal to LIMA origin (Figure 1). There was biphasic flow in the left vertebral artery, suggesting the left subclavian artery was filled by collaterals through the cerebral circulation. The LIMA was free of any disease and there was good LAD runoff (Figure 2). The right femoral artery was accessed with a 6 Fr
A 6 French (Fr) Glidesheath (Terumo) was inserted in the left radial artery. A Judkins right (JR) 4 catheter was advanced from the left radial over a J wire. The left subclavian angiogram showed a 100% chronic total occlusion (CTO) of the left SA proximal to LIMA origin (Figure 1). There was biphasic flow in the left vertebral artery, suggesting the left subclavian artery was filled by collaterals through the cerebral circulation. The LIMA was free of any disease and there was good LAD runoff (Figure 2). The right femoral artery was accessed with a 6 Fr 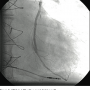 sheath. Severe native coronary artery disease was noted, with 100% occlusion of the LAD, left circumflex (LCX) and right coronary artery (RCA). The SVG to OM and SVG to RCA grafts were widely patent, with good runoff. The arch aortogram showed a very proximal 100% left subclavian artery occlusion (Figure 3). Percutaneous intervention of the left subclavian occlusion was performed to treat the lifestyle-limiting angina from coronary steal during the use of the left upper limb. From the radial access, a 0.018” Astato wire with a 30-
sheath. Severe native coronary artery disease was noted, with 100% occlusion of the LAD, left circumflex (LCX) and right coronary artery (RCA). The SVG to OM and SVG to RCA grafts were widely patent, with good runoff. The arch aortogram showed a very proximal 100% left subclavian artery occlusion (Figure 3). Percutaneous intervention of the left subclavian occlusion was performed to treat the lifestyle-limiting angina from coronary steal during the use of the left upper limb. From the radial access, a 0.018” Astato wire with a 30- gram tip weight (Asahi Intecc) was used through a 6 Fr JR 4 diagnostic catheter to insert through the distal cap of CTO. Later, a Quick cross catheter (Spectranetics) was used to support the crossing of the CTO, and the wire was inserted into the ascending aorta. The JR 4 catheter and a short 6 Fr Glidesheath were exchanged over the 0.018” wire with a 45 cm, hydrophilic-coated Ansel sheath (Cook). Guided by dual injections from the aorta (femoral access) and the radial sheath parked in distal subclavian, a 6 x 37 mm and 6 x 17 mm balloon-
gram tip weight (Asahi Intecc) was used through a 6 Fr JR 4 diagnostic catheter to insert through the distal cap of CTO. Later, a Quick cross catheter (Spectranetics) was used to support the crossing of the CTO, and the wire was inserted into the ascending aorta. The JR 4 catheter and a short 6 Fr Glidesheath were exchanged over the 0.018” wire with a 45 cm, hydrophilic-coated Ansel sheath (Cook). Guided by dual injections from the aorta (femoral access) and the radial sheath parked in distal subclavian, a 6 x 37 mm and 6 x 17 mm balloon-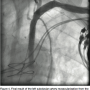 expandable stents were deployed into the subclavian. A 7 mm balloon was then used to post dilate both stents. TIMI-3 flow was re-established in the vertebral, IMA and in the LAD with an excellent final result (Figure 4). The patient had an uncomplicated hospital course and had remarkable improvement of his symptoms by the time of his clinical follow-up.
expandable stents were deployed into the subclavian. A 7 mm balloon was then used to post dilate both stents. TIMI-3 flow was re-established in the vertebral, IMA and in the LAD with an excellent final result (Figure 4). The patient had an uncomplicated hospital course and had remarkable improvement of his symptoms by the time of his clinical follow-up.
Discussion
While most patients are asymptomatic from subclavian stenosis, some patients present with symptoms of upper extremity ischemia, including arm or hand claudication with or without neurologic symptoms or symptoms of subclavian steal syndrome. Our patient presented with angina, because of coronary steal from using his left arm. After the first report of subclavian angioplasty by Bachman and Kim in 19801, percutaneous intervention of subclavian disease has grown persistently, becoming the mainstay of therapy. Endovascular treatment is less invasive and has lower complication rates when compared to surgical options. While the technical success rate is high (97%), the adverse event rate is small (6%).2 Most complications are minor, limited to vascular access difficulty and stent dislodgments.2 The choice of balloon-expandable stents versus self-expandable stents should be tailored not only to features of the lesion, but also to arterial characteristics. Balloon-expandable stents are preferred to treat a highly calcified lesion if precise deployment is required. In long lesions, tortuous vessels, and lesions that do not require precise deployment of the stent, a self-expanding stent may provide adequate radial strength with a reduced risk of stent fracture due to its innate resistance to compression.3
We always use and recommend ipsilateral radial artery access to perform diagnostic and therapeutic endovascular procedures for subclavian artery stenosis or occlusion due to the following advantages:
- The ipsilateral radial artery approach provides easy access and avoids the manipulation of catheters in the aortic arch, where the prevalence of calcified atheromatous plaques is high. This also prevents trauma and lowers the theoretic risk of embolization.
- Atherosclerotic disease of the subclavian artery commonly involves ostial and proximal portions, with a higher prevalence of proximal calcified stenosis or occlusion. The radial approach provides better support to cross through a CTO, and deliver balloons or stents. Retrograde crossing of the CTO has a higher likelihood of success. From the femoral approach, selective placement of the catheter or sheath is more challenging.
- Femoral access in the presence of peripheral vascular disease (PVD) is associated with a higher incidence of complications.4 Access site complications, including major bleeding, are significantly lower with radial access. Early ambulation reduces morbidity and patients can be discharged home immediately after removing the hemostatic radial band. The radial approach is truly an outpatient procedure and saves significant health care dollars.5
The limitations of radial access are a longer learning curve, higher chances of access failure due to ipsilateral subclavian disease, and radial artery spasm.
The following are technical tips for performing this procedure from the radial approach:
- Achieve ipsilateral radial access with a standard 5 or 6 French radial sheath.
- A JR 4 catheter and a 0.035” J wire or an angled Glidewire (Terumo) can be advanced to the subclavian artery. A distal subclavian angiogram can be performed, and guided by this angiogram, the lesion can be crossed. A standard pigtail catheter can be advanced and an arch aortogram should be performed to define any ostial disease.
- If there is occlusive disease, contralateral radial access or a 4 French femoral access can be used to perform an arch aortogram (Figure 3). To cross an occlusion, consider using a JR 4 catheter, Bernstein catheter (Boston Scientific), or an angled or straight crossing catheter in conjunction with a 0.014” or 0.018” CTO wire, per the operator’s discretion. An intraluminal course can be guided by placing a catheter from the other access. After crossing the occlusion, the intraluminal position should be confirmed. If required, a re-entry device like the Outback (Cordis), Pioneer (Medtronic) or Enteer (Covidien) can be used, as per the operator’s experience. It is relatively easy to advance these devices from the radial access to the point of disease.
- After crossing and defining the lesion over a 0.018” or 0.035” wire, the catheter and the short radial sheath can be exchanged for a 45 cm or 65 cm hydrophilic-coated sheath. If required, a support wire should be used.
- The decision to use a balloon, self-expanding stent, or balloon-expandable stent is according to the operator’s choice, based on the location and nature of the disease. Most radial arteries will accommodate only a 6 French sheath. A shaft length of 80 cm or more will be required. There are multiple commercially available stent systems that can be used with these specifications. Guided by an arch aortogram and subclavian angiogram, an image overlay or the road map tool can be used to deploy a stent or balloon from the ipsilateral radial access. In most patients, a second access will not be required. To flare the aorto-ostial stent, an oversized balloon can be inflated in the aorto-ostial location and pulled back.
- The traditional risks and complications of the subclavian intervention are the same, including dissection, perforation, plaque shift and stent migration.
Dr. Kintur Sanghvi can be contacted at SanghviK@Deborah.org.
References
- Bachman DM, Kim RM. Transluminal dilatation for subclavian steal syndrome. AJR Am J Roentgenol. 1980 Nov; 135(5): 995-996.
- Hadjipetrou P, Cox S, Piemonte T, Eisenhauer A. Percutaneous revascularization of atherosclerotic obstruction of aortic arch vessels. J Am Coll Cardiol. 1999; 33: 1238-1245.
- Amor M, Eid-Lidt G, Chati Z, Wilentz JR. Endovascular treatment of the subclavian artery: stent implantation with or without predilatation. Catheter Cardiovasc Interv. 2004; 63: 364-370.
- Samal AK, White CJ. Percutaneous management of access site complications. Catheter Cardiovasc Interv. 2002 Sep; 57(1): 12-23.
- Yu J, Korabathina R, Coppola J, Staniloae C. Transradial approach to subclavian artery stenting. J Invasive Cardiol. 2010; 22: 204-206.










