Transradial CTOs
Editor's note: View videos of CTO interventions that accompany this case report.
Can you perform chronic total occlusion (CTO) cases via both radials? If so, what size sheaths and guides are you using, and what is your preferred technique in crossing a CTO?
Case report
This is a 59-year-old male with history of hyperlipidemia and recent admission with a non-ST- elevation myocardial infarction (NSTEMI). Angiography demonstrated a chronic total occlusion (CTO) of the right coronary artery (RCA) and a high-grade stenosis of the circumflex (culprit artery). He underwent percutaneous coronary intervention (PCI) of the circumflex artery with two drug-eluting stents (Promus, Boston Scientific). Subsequent outpatient evaluation (stress) demonstrated ischemia in the RCA distribution as well as clinical symptoms. The patient was staged for revascularization of the RCA CTO.
elevation myocardial infarction (NSTEMI). Angiography demonstrated a chronic total occlusion (CTO) of the right coronary artery (RCA) and a high-grade stenosis of the circumflex (culprit artery). He underwent percutaneous coronary intervention (PCI) of the circumflex artery with two drug-eluting stents (Promus, Boston Scientific). Subsequent outpatient evaluation (stress) demonstrated ischemia in the RCA distribution as well as clinical symptoms. The patient was staged for revascularization of the RCA CTO.
Dual access was obtained with 6 French (Fr) Glidesheaths (Terumo) in both radial arteries and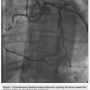 then upsized to 7 Fr sheaths, followed by administration of the radial cocktail (2500 units of heparin, 2.5 mg). From the left radial, we used an Amplatz left (AL)1 catheter with side holes to engage the RCA. From the right radial, an Extra Back-Up (EBU) 3.5 guide catheter was used to engage the left system. Simultaneous injections were performed, injecting the donor vessel first to allow better visualization of the collaterals (Figure 1). The initial strategy was to attempt antegrade crossing first and switch to a retrograde approach if unsuccessful. We then administered heparin to an activated clotting time (ACT) >250. A Fielder XT wire (Abbott Vascular) and an over-the-wire 1.5 x 8mm Emerge balloon (Boston Scientific) were used to wire the RCA and it was felt we entered the subintimal space. We left this wire in the false lumen and
then upsized to 7 Fr sheaths, followed by administration of the radial cocktail (2500 units of heparin, 2.5 mg). From the left radial, we used an Amplatz left (AL)1 catheter with side holes to engage the RCA. From the right radial, an Extra Back-Up (EBU) 3.5 guide catheter was used to engage the left system. Simultaneous injections were performed, injecting the donor vessel first to allow better visualization of the collaterals (Figure 1). The initial strategy was to attempt antegrade crossing first and switch to a retrograde approach if unsuccessful. We then administered heparin to an activated clotting time (ACT) >250. A Fielder XT wire (Abbott Vascular) and an over-the-wire 1.5 x 8mm Emerge balloon (Boston Scientific) were used to wire the RCA and it was felt we entered the subintimal space. We left this wire in the false lumen and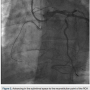 tried to advance a MiracleBros 6 wire (Abbott Vascular) with the over-the-wire balloon; however, that found the false lumen as well. Utilizing the CrossBoss and Stingray reentry system (Boston Scientific), we advanced in the subintimal space to the reconstitution point of the RCA (Figure 2). Contralateral injection demonstrated poor visualization of the distal vessel, likely from antegrade flow (from the subintimal dissection) compressing the vessel (compare distal RCA filling in Figures 1-2). Rather than further extend the dissection plane distally and attempt re-entry, it was decided to proceed with the retrograde approach. After administering additional
tried to advance a MiracleBros 6 wire (Abbott Vascular) with the over-the-wire balloon; however, that found the false lumen as well. Utilizing the CrossBoss and Stingray reentry system (Boston Scientific), we advanced in the subintimal space to the reconstitution point of the RCA (Figure 2). Contralateral injection demonstrated poor visualization of the distal vessel, likely from antegrade flow (from the subintimal dissection) compressing the vessel (compare distal RCA filling in Figures 1-2). Rather than further extend the dissection plane distally and attempt re-entry, it was decided to proceed with the retrograde approach. After administering additional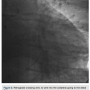 heparin to achieve an ACT >300, we went from the left system with a Runthrough wire (Terumo) and an over-the-wire balloon, using the workhorse wire to wire the circumflex. We then changed to a retrograde crossing wire, the Asahi Fielder FC (Abbott Vascular), to wire into the collateral going to the distal RCA (Figure 3) and were able to advance in a retrograde fashion all the way into the true RCA (Figure 4). We backed out the 1.5 mm balloon and trapped it in the guide with a 2.5 x 20 Emerge (Boston Scientific), inflated to 16 atmospheres. We exchanged out initially for a Corsair catheter (Asahi Intecc) and advanced retrograde into the
heparin to achieve an ACT >300, we went from the left system with a Runthrough wire (Terumo) and an over-the-wire balloon, using the workhorse wire to wire the circumflex. We then changed to a retrograde crossing wire, the Asahi Fielder FC (Abbott Vascular), to wire into the collateral going to the distal RCA (Figure 3) and were able to advance in a retrograde fashion all the way into the true RCA (Figure 4). We backed out the 1.5 mm balloon and trapped it in the guide with a 2.5 x 20 Emerge (Boston Scientific), inflated to 16 atmospheres. We exchanged out initially for a Corsair catheter (Asahi Intecc) and advanced retrograde into the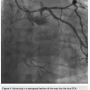 RCA (Figure 5). Using a Pilot wire (Abbott Vascular), we were able to advance in a retrograde fashion into the RCA guide, but the Corsair catheter was not long enough to go all the way (a 135 cm catheter was inadvertently opened instead of the 150 cm). Therefore, we trapped the Pilot wire into the RCA guide with a 2.5 x 20 Emerge balloon after removing the antegrade wire (the Pilot wire is hydrophilic; therefore, one needs to exercise caution when trapping). We removed the 135 cm Corsair and advanced a 150 cm Corsair retrograde into the RCA guide. We then removed the Pilot wire through the Corsair, running retrograde from the left guide to the right guide. We rewired the Corsair in an antegrade fashion, rather than
RCA (Figure 5). Using a Pilot wire (Abbott Vascular), we were able to advance in a retrograde fashion into the RCA guide, but the Corsair catheter was not long enough to go all the way (a 135 cm catheter was inadvertently opened instead of the 150 cm). Therefore, we trapped the Pilot wire into the RCA guide with a 2.5 x 20 Emerge balloon after removing the antegrade wire (the Pilot wire is hydrophilic; therefore, one needs to exercise caution when trapping). We removed the 135 cm Corsair and advanced a 150 cm Corsair retrograde into the RCA guide. We then removed the Pilot wire through the Corsair, running retrograde from the left guide to the right guide. We rewired the Corsair in an antegrade fashion, rather than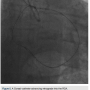 externalizing it in the left radial, through the RCA guide (this does sacrifice the ultimate rail for delivery of balloons or stents by eliminating the externalized wire). The floppy tip of the Pilot wire was now in the distal vessel (similar to standard antegrade wiring of the vessel) and we backed out the Corsair (Figure 6). We advanced a 2.5 x 30 mm balloon and predilated. Intravascular ultrasound (IVUS) (Boston Scientific) demonstrated the RCA was a fairly large vessel and therefore, a 4.0 x 38mm Promus drug-eluting stent was placed in the mid RCA. The distal lesion was treated. We dilated with a 3.0 x 30 mm balloon and noticed a distal dissection.
externalizing it in the left radial, through the RCA guide (this does sacrifice the ultimate rail for delivery of balloons or stents by eliminating the externalized wire). The floppy tip of the Pilot wire was now in the distal vessel (similar to standard antegrade wiring of the vessel) and we backed out the Corsair (Figure 6). We advanced a 2.5 x 30 mm balloon and predilated. Intravascular ultrasound (IVUS) (Boston Scientific) demonstrated the RCA was a fairly large vessel and therefore, a 4.0 x 38mm Promus drug-eluting stent was placed in the mid RCA. The distal lesion was treated. We dilated with a 3.0 x 30 mm balloon and noticed a distal dissection.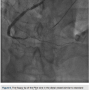 There was transient loss of flow in the posterior left ventricular branch (PLV), which was wired with a Fielder XT wire, and then changed out for a Pilot wire through an over-the-wire balloon (Figure 7). We exchanged for a Runthrough wire into the distal posterior descending coronary artery (PDA). The Corsair was removed from the collateral, and we took the guide and wire from the left system. Two additional 3.0 x 28 mm Promus drug-eluting stents were placed distally prior to the bifurcation. After stent placement, there was good flow in the distal vessel. A gap in between the stents was covered with a 3.0 x 8 mm Promus drug-eluting stent. We then
There was transient loss of flow in the posterior left ventricular branch (PLV), which was wired with a Fielder XT wire, and then changed out for a Pilot wire through an over-the-wire balloon (Figure 7). We exchanged for a Runthrough wire into the distal posterior descending coronary artery (PDA). The Corsair was removed from the collateral, and we took the guide and wire from the left system. Two additional 3.0 x 28 mm Promus drug-eluting stents were placed distally prior to the bifurcation. After stent placement, there was good flow in the distal vessel. A gap in between the stents was covered with a 3.0 x 8 mm Promus drug-eluting stent. We then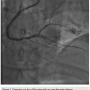 post dilated with a 3.25 x 15 mm Quantum balloon (Boston Scientific) at high pressure (Figures 8-9). Because of a pseudo lesion in the proximal vessel, we exchanged out for a Judkins right (JR) 4 guide and gave 200 mcg intracoronary (IC) nitroglycerin. Angiography revealed TIMI-3 flow in the vessel without any perforation or embolization (Figure 10). The patient tolerated the procedure well. The guide was removed and a TR Band (Terumo) was placed over bilateral radials. The patient has done well clinically, and is approximately 6 months post PCI with excellent functional capacity and no ischemic symptoms.
post dilated with a 3.25 x 15 mm Quantum balloon (Boston Scientific) at high pressure (Figures 8-9). Because of a pseudo lesion in the proximal vessel, we exchanged out for a Judkins right (JR) 4 guide and gave 200 mcg intracoronary (IC) nitroglycerin. Angiography revealed TIMI-3 flow in the vessel without any perforation or embolization (Figure 10). The patient tolerated the procedure well. The guide was removed and a TR Band (Terumo) was placed over bilateral radials. The patient has done well clinically, and is approximately 6 months post PCI with excellent functional capacity and no ischemic symptoms.
Discussion
Chronic total occlusions (CTOs) occur approximately 20-30% of the time during diagnostic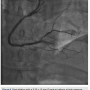 angiography1; however, revascularization is only attempted in a minority of cases. In the BARI study2, revascularization was attempted only 8-15% of the time and the presence of a CTO was the most common (68%) angiographic exclusion criteria leading to bypass surgery.3-6 Rationale for CTO recanalization includes relief of angina, improvement of left ventricular function, to obviate the need for bypass, and improved survival.7-9 Multiple studies have shown improved long-term survival in patients in whom the recanalization procedure was successful, as compared to failed attempt.10-12
angiography1; however, revascularization is only attempted in a minority of cases. In the BARI study2, revascularization was attempted only 8-15% of the time and the presence of a CTO was the most common (68%) angiographic exclusion criteria leading to bypass surgery.3-6 Rationale for CTO recanalization includes relief of angina, improvement of left ventricular function, to obviate the need for bypass, and improved survival.7-9 Multiple studies have shown improved long-term survival in patients in whom the recanalization procedure was successful, as compared to failed attempt.10-12
A full discussion of CTOs is beyond the scope of the article and can be found in various textbook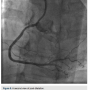 and online resources. This article highlights some of the technical considerations and limitations when utilizing the radial approach for CTO recanalization. Many techniques have been described for crossing CTOs; however, there are two basic strategies: antegrade or retrograde. Success rates have varied, depending on the chosen strategy and operator experience, with overall historical success of 50-70%. A hybrid algorithm utilizing both approaches has led to decreased procedure times and increased crossing success (for a full description, visit https://www.ctofundamentals.org).
and online resources. This article highlights some of the technical considerations and limitations when utilizing the radial approach for CTO recanalization. Many techniques have been described for crossing CTOs; however, there are two basic strategies: antegrade or retrograde. Success rates have varied, depending on the chosen strategy and operator experience, with overall historical success of 50-70%. A hybrid algorithm utilizing both approaches has led to decreased procedure times and increased crossing success (for a full description, visit https://www.ctofundamentals.org).
Many CTO operators utilize an 8 Fr system as their default and thus, must use the femoral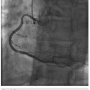 approach. This allows more passive guide support, usage of over-the-wire systems (support/crossing catheters, over-the-wire balloons) and simultaneous IVUS, and easy “trapping” of gear. This does not mean that smaller systems (6 or 7 Fr) or use of radial access have no utility in approaching CTOs. Observational studies have demonstrated equivalent success with transradial access (in experienced radial operators) versus transfemoral access, with suggested lower bleeding complications with radial access use.13,14
approach. This allows more passive guide support, usage of over-the-wire systems (support/crossing catheters, over-the-wire balloons) and simultaneous IVUS, and easy “trapping” of gear. This does not mean that smaller systems (6 or 7 Fr) or use of radial access have no utility in approaching CTOs. Observational studies have demonstrated equivalent success with transradial access (in experienced radial operators) versus transfemoral access, with suggested lower bleeding complications with radial access use.13,14
Utilizing the radial approach for a CTO usually limits the operator to either a 6 or 7 Fr guide. There are certain limitations to consider with the smaller system, as it may alter strategy or access. As we (currently a high-volume radial operator utilizing the radial approach in 90-95% of coronary PCIs) have adopted the hybrid algorithm as our approach to CTOs, we initially started with a femoral approach. As the case volume increased, and a level of comfort developed with the various devices and techniques, we have been utilizing bilateral radial access with either dual 7 Fr access, or one 6 and one 7 Fr, depending on the patient and the coronary anatomy.
The first limitation involves the guide: guide size and guide support. As we noted earlier, most cases will be done through either a 6 or 7 Fr system. Unlike with larger lumen guides, knowledge of various device sizes and materials is required. Different manufacturers may use the same material, but have different profiles for balloons (not allowing kissing balloons in a 6 Fr guide, for example). An alternative is to consider the sheathless guides, which may allow use of a larger lumen. Length of the guide is also important. For a retrograde approach, a 90 cm guide may be necessary to allow externalization of the retrograde wire. This not as a much of a concern with regular 6 and 7 Fr guides; however, sheathless guides are currently not available in short lengths. Alternative solutions include a guide cut down or high radial stick. Guide support is also a consideration. In general, larger guides will offer more passive support. Anatomical variations between the access point and the coronaries may affect the torque and support of the catheter as well.15 Utilization of the “opposite” radial access to improve support (left radial for the right coronary and right radial for left coronary) has been suggested.14
Gear manipulation is another concern. With a 6 Fr system, there are limitations in the ability to use certain techniques. For example, one cannot “trap” in a 6 French system (meaning inflate a balloon in the guide to pin a wire, and allow removal of an over-the-wire balloon or catheter). This could be problematic when trying to maintain wire position, and remove or advance a different support catheter or re-entry device. Use of a 300 cm wire is a solution, but runs the risk of losing wire position. Use of the Guideliner (Vascular Solutions) or Guidezilla (Boston Scientific) catheters may be limited as well. There are 6 Fr versions, but these cannot be utilized for trapping or use of a buddy wire.
Conclusion
Radial access is an alternative access for CTOs with equivalent success rates compared to femoral access in experienced operators. Dual injection is recommended for adequate visualization of the CTO length, as well as visualization of the collaterals (septals or epicardials) for the retrograde approach. When adopting the hybrid algorithm, it is probably better to start with femoral access and large-caliber guides until one is comfortable with this approach and its variations. After overcoming the learning curve for both radial and CTOs, is then reasonable to transition to a radial approach. For more information about CTOs and the hybrid algorithm, visit https://www.ctofundamentals.org.
Disclosure: Orlando Marrero reports no conflicts of interest regarding the content herein. Dr. Zaheed Tai reports the following: Terumo (proctor for transradial course), Spectranetics (proctor for laser course, speaker, advisory board), Medicines Company (speakers bureau).
Orlando Marrero can be contacted at orlm8597@yahoo.com. Dr. Zaheed Tai can be contacted at zaheedtai@gmail.com.
References
- Kahn JK. Angiographic suitability for catheter revascularization of total coronary occlusions in patients from a community hospital setting. Am Heart J. 1993; 126: 561-564.
- Srinivas VS, Brooks MM, Detre KM, King SB III, Jacobs AK, Johnston J, Williams DO. Contemporary percutaneous coronary intervention versus balloon angioplasty for multivessel coronary artery disease: A comparison of the National Heart, Lung and Blood Institute Dynamic Registry and the Bypass Angioplasty Revascularization Investigation (BARI) study. Circulation. 2002; 106: 1627-1633.
- Anderson HV, Shaw RE, Brindis RG, Hewitt K, Krone RJ, Block PC, McKay CR, Weintraub WS. A contemporary overview of percutaneous coronary interventions. The American College of Cardiology-National Cardiovascular Data Registry (ACC-NCDR). J Am Coll Cardiol. 2002; 39: 1096-1103.
- Williams DO, Holubkov R, Yeh W, Bourassa MG, Al-Bassam M, et al. Percutaneous coronary intervention in the current era compared with 1985-1986: The National Heart, Lung, and Blood Institute Registries. Circulation. 2000; 102: 2945-2951.
- Bourassa MG, Roubin GS, Detre KM, Sopko G, Krone RJ, Attabuto MJ, Bjerregaad P, Bolling , S. Herman MV, Frye R. Bypass angioplasty revascularization investigation: Patient screening, selection, and recruitment. Am J Cardiol. 1995; 75: 3C-8C.
- Delacretaz E, Meier B. Therapeutic strategy with total coronary artery occlusions. Am J Cardiol. 1997; 79: 185-187.
- Warren RJ, Black AJ, Valentine PA, Manolas EG, Hunt D. Coronary angioplasty for chronic total occlusion reduces the need for subsequent coronary bypass surgery. Am Heart J. 1990; 120: 270–274.
- Ivanhoe RJ, Weintraub WS, Douglas JS, Lembo NJ, Furman M, Gershony G, Cohen CL, King SB. Percutaneous transluminal coronary angioplasty of chronic total occlusions. Primary success, restenosis, and long-term clinical follow-up. Circulation. 1992; 85: 106-115.
- Suero J, Marso S, Jones P, Laster S, Huber K, Giorgi L, John- son W, Rutherford B. Procedural outcomes and long-term survival among patients undergoing percutaneous coronary intervention of a chronic total occlusion in native coronary arteries: A 20-year experience. J Am Coll Cardiol. 2001; 38: 409-414.
- Olivari Z, Rubartelli P, Piscione F, Ettori F, Fontanelli A, et al. Immediate results and one-year clinical outcome after percutaneous coronary interventions in chronic total occlusions: Data from a multicenter, prospective, observational study (TOAST-GISE). J Am Coll Cardiol. 2003; 41: 1672-1678.
- Hoye A, van Domburg RT, Sonnenschein K, Serruys PW. Percutaneous coronary intervention for chronic total occlusions: The Thoraxcenter experience 1992-2002. Eur Heart J. 2005; 26: 2630-2636.
- Khan MF, Wendel CS, Thai HM, Movahed MR. Effects of percutaneous revascularization of chronic total occlusions on clinical outcomes: A meta-analysis comparing successful versus failed percutaneous intervention for chronic total occlusion. Cathet Cardiovasc Intervent. 2013; 82: 95-107. doi: 10.1002/ccd.24863
- Rathore S, Hakeem A, Pauriah M, Roberts E, Beaumont A, Morris JL. A comparison of the transradial and the transfemoral approach in chronic total occlusion percutaneous coronary intervention. Catheter Cardiovasc Interv. 2009; 73: 883-887.
- Burzotta F, De Vita M, Lefevre T, Tommasino A, Louvard Y, Trani C. Radial approach for percutaneous coronary interventions on chronic total occlusions: Technical issues and data review. Cathet Cardiovasc Intervent. 2013 Jul 5:0. doi: 10.1002/ccd.25118.
- Burzotta F, Trani C, De Vita M, Crea F. A new operative classification of both anatomic vascular variants and physiopathologic conditions affecting transradial cardiovascular procedures. Int J Cardiol. 2010; 145: 120-122.










