A TCT Breakfast Symposium Summary: Imaging STEMI Culprit Plaques with Near-Infrared Spectroscopy
Presentations across the 2012 TCT meeting highlighted intravascular imaging of plaque composition, emphasizing its importance and ability to aid in the treatment of coronary artery disease. A breakfast symposium held October 25th during TCT discussed the ability of near-infrared spectroscopy to identify lipid core plaque1 and focused on the resulting clinical implications.
About near-infrared spectroscopy (NIRS)
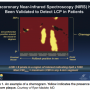 Imaging with NIRS (Infraredx, Inc.) produces a chemogram, or two-dimensional map of the artery that indicates the location of lipid core plaque (Figure 1). Red indicates the absence of lipid core plaque and yellow indicates the presence of lipid core plaque at any given site. On the chemogram, the x-axis represents millimeters of pullback in the artery and the y-axis represents the circumferential position around the vessel in degrees. The proportion of pullback positive for lipid core plaque can be quantified via the lipid core burden index (LCBI). Additional derived parameters, such as maxLCBI(4mm) in a region of interest can be used to assess specific risks. For example, a maxLCBI(4mm)≥500 corresponds to a large lipid core plaque which is 4mm long and occupies at least 180° of the vessel circumference,2 and portends a 12-fold risk increase for peri-procedural MI. NIRS is now utilized in combination with spatially co-registered IVUS for assessment of both vessel structure and chemical composition (NIRS-IVUS, TVC Imaging System, Infraredx).
Imaging with NIRS (Infraredx, Inc.) produces a chemogram, or two-dimensional map of the artery that indicates the location of lipid core plaque (Figure 1). Red indicates the absence of lipid core plaque and yellow indicates the presence of lipid core plaque at any given site. On the chemogram, the x-axis represents millimeters of pullback in the artery and the y-axis represents the circumferential position around the vessel in degrees. The proportion of pullback positive for lipid core plaque can be quantified via the lipid core burden index (LCBI). Additional derived parameters, such as maxLCBI(4mm) in a region of interest can be used to assess specific risks. For example, a maxLCBI(4mm)≥500 corresponds to a large lipid core plaque which is 4mm long and occupies at least 180° of the vessel circumference,2 and portends a 12-fold risk increase for peri-procedural MI. NIRS is now utilized in combination with spatially co-registered IVUS for assessment of both vessel structure and chemical composition (NIRS-IVUS, TVC Imaging System, Infraredx).
Using NIRS to identify ST-elevation myocardial infarction (STEMI) culprit plaques: A case control study
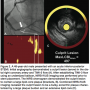 Autopsy data have shown that the majority of myocardial infarctions are attributable to rupture of a lipid core plaque. Ryan D. Madder, MD, of the Frederik Meijer Heart & Vascular Institute, Spectrum Health System, Grand Rapids, Michigan, presented a single-center, case control analysis. The analysis compared NIRS findings at STEMI culprit sites to NIRS findings of 4 control groups (including non-culprit segments in the STEMI culprit artery, and 3 autopsy controls: specimens with and without large lipid core plaques, and those with no clinical history of coronary artery disease in their lifetime). IVUS measurements and statistical analysis were performed at the Atherosclerosis Imaging Core Laboratory of the Cleveland Clinic.
Autopsy data have shown that the majority of myocardial infarctions are attributable to rupture of a lipid core plaque. Ryan D. Madder, MD, of the Frederik Meijer Heart & Vascular Institute, Spectrum Health System, Grand Rapids, Michigan, presented a single-center, case control analysis. The analysis compared NIRS findings at STEMI culprit sites to NIRS findings of 4 control groups (including non-culprit segments in the STEMI culprit artery, and 3 autopsy controls: specimens with and without large lipid core plaques, and those with no clinical history of coronary artery disease in their lifetime). IVUS measurements and statistical analysis were performed at the Atherosclerosis Imaging Core Laboratory of the Cleveland Clinic.
NIRS-IVUS was performed in STEMI culprit segments after TIMI-3 flow was established by either balloon angioplasty using an undersized balloon or aspiration thrombectomy. NIRS imaging was performed prior to stent placement in all patients.
In 19 of the 20 STEMI culprit segments, NIRS documented lipid core plaque concentrated at the culprit site. “In many of these cases,” Madder said, “the remainder of the vessel was relatively free of lipid. In addition, a lot of these plaques are nearly circumferential around the vessel wall.”
He concluded by presenting the case of documented lipid core plaque rupture resulting in sudden cardiac arrest in an otherwise healthy marathon runner during a race. “We believe this signal is very likely to be present in the artery before the event occurs, but a larger, prospective study is needed to test this hypothesis that NIRS can provide accurate, site-specific prediction that a given plaque is likely to cause a coronary event,” he said. This case, said Madder, is representative of the possibility that “rupture of a lipid core plaque likely accounts for a significant proportion of sudden cardiac death cases.”
“Yellow means trouble”: two cases from the early Swedish experience with NIRS-IVUS guided PCI
 David Erlinge, MD, PhD, Lund University Hospital, Lund, Sweden, presented one of his earliest cases using NIRS technology, a 56-year-old woman with an acute anterior STEMI. The patient had TIMI-1 flow in the mid left anterior descending coronary artery (LAD), with a large plaque just before the bifurcation to the diagonal. The patient received a stent and the distal edge landed in the midst of a lipid core plaque (Figure 3), causing a dissection. The dissection was stented with a good result. The proximal part of the first stent was then post-dilated with 3.5x15mm balloon. Another large lipid core plaque proximal to the stent (Figure 3) ruptured as a result. A third stent was then placed in the proximal LAD, and at this point, a good final result was achieved. The chemogram also shows the lipid core in the area is gone (Figure 3). Dr. Erlinge reflected that had he taken the NIRS chemogram into consideration while developing his stenting strategy for this case, he would have used a longer stent initially, covering all the yellow lipid core to avoid problems. “That was the first case that I think actually gave me some insight as to what these yellow plaques mean to us when we do PCI,” he said.
David Erlinge, MD, PhD, Lund University Hospital, Lund, Sweden, presented one of his earliest cases using NIRS technology, a 56-year-old woman with an acute anterior STEMI. The patient had TIMI-1 flow in the mid left anterior descending coronary artery (LAD), with a large plaque just before the bifurcation to the diagonal. The patient received a stent and the distal edge landed in the midst of a lipid core plaque (Figure 3), causing a dissection. The dissection was stented with a good result. The proximal part of the first stent was then post-dilated with 3.5x15mm balloon. Another large lipid core plaque proximal to the stent (Figure 3) ruptured as a result. A third stent was then placed in the proximal LAD, and at this point, a good final result was achieved. The chemogram also shows the lipid core in the area is gone (Figure 3). Dr. Erlinge reflected that had he taken the NIRS chemogram into consideration while developing his stenting strategy for this case, he would have used a longer stent initially, covering all the yellow lipid core to avoid problems. “That was the first case that I think actually gave me some insight as to what these yellow plaques mean to us when we do PCI,” he said.
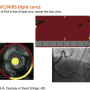 The second case was a 67-year-old male whose father died of an MI at 52 years of age. The patient presented with severe chest pain and elevated troponin, but had a normal LAD and circumflex, with no atherosclerosis. The distal RCA showed a 30% stenosis with haziness. By grayscale IVUS, this lesion had a plaque burden of 61% and a minimum lumen area (MLA) of 4mm2, which by PROSPECT Study criteria indicated that is was not at significant risk for a future event. ”It was in a part of the vessel that had a lot of movement,” Erlinge noted, ”so that could affect the risk of developing plaques. But,” he asked, ”would you stent this vessel? It was definitely not significant on the angiogram. I think if I did an FFR [fractional flow reserve], it would be negative.” NIRS showed yellow lipid core plaque at the stenosis site (Figure 4) and a 3.5 mm stent was placed. The patient was discharged the next day and has been free from chest pain for several months. ”To me, this was clear evidence that it was a vulnerable plaque that needed treatment. I think that [NIRS] helped with decision-making,” Erlinge concluded.
The second case was a 67-year-old male whose father died of an MI at 52 years of age. The patient presented with severe chest pain and elevated troponin, but had a normal LAD and circumflex, with no atherosclerosis. The distal RCA showed a 30% stenosis with haziness. By grayscale IVUS, this lesion had a plaque burden of 61% and a minimum lumen area (MLA) of 4mm2, which by PROSPECT Study criteria indicated that is was not at significant risk for a future event. ”It was in a part of the vessel that had a lot of movement,” Erlinge noted, ”so that could affect the risk of developing plaques. But,” he asked, ”would you stent this vessel? It was definitely not significant on the angiogram. I think if I did an FFR [fractional flow reserve], it would be negative.” NIRS showed yellow lipid core plaque at the stenosis site (Figure 4) and a 3.5 mm stent was placed. The patient was discharged the next day and has been free from chest pain for several months. ”To me, this was clear evidence that it was a vulnerable plaque that needed treatment. I think that [NIRS] helped with decision-making,” Erlinge concluded.
James Goldstein, MD, Director of Research and Education, William Beaumont Hospital, Royal Oak, Michigan, commented, “[The second case] shows why looking at FFR and flow limitations doesn’t give you insight as to what is happening with the biology of the plaque. It’s not just about flow.”
The role of NIRS-IVUS guided PCI in interventional practice
Interventionalists need to look beyond angiography in four key areas, said Dr. Goldstein: 1) measuring optimal stent length, 2) characterizing lesions at embolic risk, 3) optimizing stent deployment, and 4) determining vulnerable plaque. IVUS has been valuable in determining stent sizing, and ensuring proper stent placement, apposition, and avoidance of edge problems. Yet while IVUS can determine plaque presence and severity, it has limited ability to characterize plaque composition. “Lipid core plaque behaves differently than fibrous plaque,” said Dr. Goldstein. He noted that lipid core plaque:
- Has a more rapid progression;
- Is the proximate cause of an unstable plaque;
- Is prone to embolization during stenting;
- Poses a higher risk for subacute and late stent thrombosis.
As Dr. Erlinge demonstrated in his first case, stenting in the midst of a lipid core plaque can also lead to edge dissections.
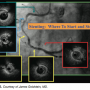 While discrete lesions do occur, often the disease that presents in the cath lab is far more ambiguous. As James Muller, MD, founder and chief medical officer of Infraredx, commented earlier, “The biggest problem with coronary disease is not treatment, but diagnosis.” Dr. Goldstein presented the example of a patient with chronic stable angina and a positive stress test with inferior wall motion on the stress echo. The right coronary artery is diffusely diseased, as seen by angiography (Figure 5). “Where do you start? Where do you stop?” he said. By IVUS, the right coronary artery shows the presence of plaque:
While discrete lesions do occur, often the disease that presents in the cath lab is far more ambiguous. As James Muller, MD, founder and chief medical officer of Infraredx, commented earlier, “The biggest problem with coronary disease is not treatment, but diagnosis.” Dr. Goldstein presented the example of a patient with chronic stable angina and a positive stress test with inferior wall motion on the stress echo. The right coronary artery is diffusely diseased, as seen by angiography (Figure 5). “Where do you start? Where do you stop?” he said. By IVUS, the right coronary artery shows the presence of plaque:
- Distally;
- In a portion of the vessel that looks “normal” by angiography;
- In a long segment of disease with tightly packed plaque;
- In a more proximal, eccentric lesion, heavily laden with plaque;
- Proximally.
“Do we stent too little? Stent too much?” he continued. “Angiography alone is very limiting. I think we can do better. How we do this may impact outcomes.”
A second example was the case of a 55-year-old man with a progressive stress test demonstrating anterior ischemia. An LAD lesion was the culprit by angiography. The lesion was stented and ballooned with good angiographic results. Four months later, the patient returned with recurrent symptoms and a positive stress test, again with the same results. Angiography showed the stented area was patent, but just proximal to the stent was nearly occluded. If the patient had occluded, the result most likely would have been sudden death or a massive anterior MI. The patient went on to have successful bypass surgery. “Could what might have been a calamitous problem have been prevented by doing direct coronary imaging and assuring that the full length of the lesion was covered, or in fact if it extended into the left main?” asked Dr. Goldstein.
The question of how often, by angiography alone, would lipid core plaque be missed was studied by Simon Dixon et al.3 A retrospective study of 83 patients compared an individual estimation of length of vessel to stent by angiography against the same intended stent zone with lipid core plaque shown by NIRS. Two-thirds of the lesions in the study showed lipid core plaque. Within this group, in at least one out of every five cases, lipid core plaque extended beyond what the operator would have treated with angiogram alone.
Plaque composition can also contribute to adverse coronary events during PCI. Percutaneous coronary intervention is associated with peri-procedural myocardial infarction (MI) in 3-15% of cases. In many cases, these MIs result from distal embolization of lipid-core plaques. MRI has shown that many of these patients have myocardial fibrosis and it has been associated with increased morbidity and mortality over time. “These are not trivial events,” noted Dr. Goldstein. He presented a case of an unstable acute coronary syndrome patient with an ulcerated plaque in the right coronary artery. NIRS showed a large, circumferential lipid core plaque (similar to what Dr. Madder showed in his series of STEMI cases). After the plaque underwent balloon dilatation, there was severe no-reflow. The patient became extremely ill and required resuscitation. “Large, lipid core plaques put patients at high risk,” cautioned Dr. Goldstein. “A maxLCBI(4mm)>500 means you have a 50% likelihood of having a periprocedural MI.”2,4
Dr. Goldstein then returned to the question raised by Dr. Madder in his case of the marathon runner who experienced sudden cardiac arrest. “Can we find these vulnerable plaques prospectively and come up with a strategy, perhaps preemptive stenting, to be proven in a prospective trial?” he asked.
He concluded that while angiographically-guided PCI provides good results and outcomes, it comes with attendant risks of undersized and/or underexpanded stents, missed edge dissections, and uncovered plaque, particularly lipid core plaque. NIRS-IVUS guided PCI provides guidance regarding both plaque composition and architecture. IVUS measures minimum lumen diameter, provides guidance on stent length, and allows the operator to ensure optimal stent expansion and avoid stent edge complications. NIRS also aids in determining the length of the vessel to stent, and allows the operator to calculate distal embolization risk and visualize plaque vulnerability.
Q&A: Suggestions on how to treat
“When you see these [lipid core plaques] in a non-culprit segment of the vessel, what do you do?” asked Dr. Madder. When he discovers a high volume of lipid core plaque, Dr. Madder said, those patients are more likely to receive high-dose statin therapy.
“Maybe we should send that patient home on statins for six weeks, and then stent the lesion to avoid periprocedural MI,” suggested Dr. Erlinge. However, he said, patients with large lipid core plaques certainly could be stented with an absorbable stent, a drug-eluting balloon, or a special stent that doesn’t allow lipid penetration. NIRS-IVUS has changed his practice, said Dr. Erlinge. If he sees a patient with a high number of lipid core plaques throughout the vessels, for example, he makes certain that the patient goes on high-dose statin therapy and lowers LDL levels.
If a non flow-limiting, large lipid core plaque is identified, “is the treatment pharmacologic, or is it local and interventional?” asked Dr. Muller. “If a person is full of yellow plaque, you might want to give them stronger antiplatelet therapy or more intense anti-lipid therapy. Dr. Renu Virmani has questioned whether any pharmacologic therapy will work on a lipid core that is calcified or fibrotic. Is it like an abscess? You can’t cure an abscess with an antibiotic. You have to incise and drain it.”
Prospective clinical trials to provide guidance on these important clinical questions are being planned. Dr. Goldstein also emphasized that even though the guidelines do not include NIRS intracoronary imaging at present, that interventionalists should not ignore the information provided by NIRS. “Deciding to do nothing is still a decision, and we need to take responsibility for that,” he said.
A question arose as to what exactly happens to the yellow lipid core plaque when it disappears on the chemogram after stenting. Dr. Erlinge responded, “It seems like since you can collect it in a distal embolization device, that it goes distally in the artery and probably to the microcirculation.”
Sean Madden, principal scientist with Infraredx, also responded. “We have all possible outcomes to this question represented in the COLOR registry5,” he said. “We have some lesions that don’t change — maybe these are thick capped. We have some lesions where the lipid nearly goes away — these might be very thin-capped plaques, where the contents were completely pressed out and released.
Learn more about near-infrared spectroscopy at www.infraredx.com.
References and recommended reading
- Gardner CM, Tan H, Hull EL, et al. Detection of lipid core coronary plaques in autopsy specimens with a novel catheter-based near-infrared spectroscopy system. J Am Coll Cardiol Img. 2008;1(5):638-648.
- Goldstein JA, Maini B, Dixon SR, Brilakis ES, Grines CL, Rizik DG, et al. Detection of lipid-core plaques by intracoronary near-infrared spectroscopy identifies high risk of periprocedural myocardial infarction. Circ Cardiovasc Interv. 2011 Oct 1; 4(5): 429-437.
- Dixon SR, Grines CL, Munir A, Madder RD, Safian RD, Hanzel GS, Pica MC, Goldstein JA. Analysis of target lesion length before coronary artery stenting using angiography and near-infrared spectroscopy versus angiography alone. Am J Cardiol. 2012 Jan 1;109(1):60-66.
- Brilakis ES, Abdel-Karim AR, Papayannis AC, Michael TT, Rangan BV, Johnson JL, Banerjee S. Embolic protection device utilization during stenting of native coronary artery lesions with large lipid core plaques as detected by near-infrared spectroscopy. Catheter Cardiovasc Interv. 2012 Apr 17. doi: 10.1002/ccd.23507.
- Clinicaltrials.gov. A listing of clinical trials by the search term “Infraredx”. Available online at https://www.clinicaltrials.gov/ct2/results?term=infraredx. Accessed November 13, 2012.
- Madder RD, Steinberg DH, Anderson RD. Multimodality direct coronary imaging with combined near-infrared spectroscopy and intravascular ultrasound: Initial US experience. Catheter Cardiovasc Interv. 2011 Nov 22. doi: 10.1002/ccd.23358.










