Pulmonary Embolism: The SEATTLE II Trial
How do you perform catheter-directed ultrasound thrombolysis?
 We use a specially designed catheter, the EkoSonic Endovascular System (Ekos Corporation), composed of two components. The first component is a catheter that fits through a 6 French sheath, and usually placed in the femoral vein. It is passed over an .035” guide wire, up the inferior vena cava into the right atrium. It crosses the tricuspid valve into the right ventricle and goes up through the right ventricular outflow tract into the pulmonary arteries. The first portion of the catheter has multiple side holes that allow for the delivery of a thrombolytic agent, such as tPA. The second portion of the catheter is a central ultrasonic core wire. Each transducer is very small and is placed about 1 cm apart, and this portion sits inside the first catheter with the side holes. The ultrasonic core vibrates at 2 million cycles per second, or 2 megahertz. The resulting ultrasonic energy breaks up clot by exposing plasminogen receptor sites, making the clot more ‘porous’, so to speak, and allowing delivery of tPA drug into the clot. The ultrasonic waves drive the drug deeper inside the clot. It also allows us to use less tPA, meaning a lower risk of bleeding complications.
We use a specially designed catheter, the EkoSonic Endovascular System (Ekos Corporation), composed of two components. The first component is a catheter that fits through a 6 French sheath, and usually placed in the femoral vein. It is passed over an .035” guide wire, up the inferior vena cava into the right atrium. It crosses the tricuspid valve into the right ventricle and goes up through the right ventricular outflow tract into the pulmonary arteries. The first portion of the catheter has multiple side holes that allow for the delivery of a thrombolytic agent, such as tPA. The second portion of the catheter is a central ultrasonic core wire. Each transducer is very small and is placed about 1 cm apart, and this portion sits inside the first catheter with the side holes. The ultrasonic core vibrates at 2 million cycles per second, or 2 megahertz. The resulting ultrasonic energy breaks up clot by exposing plasminogen receptor sites, making the clot more ‘porous’, so to speak, and allowing delivery of tPA drug into the clot. The ultrasonic waves drive the drug deeper inside the clot. It also allows us to use less tPA, meaning a lower risk of bleeding complications.
Bleeding complications are an issue with the traditional systemic dose of tPA in acute pulmonary embolism.
Exactly. That is primarily why physicians have been very reluctant to use systemic tPA. There is the elevated incidence of intracranial bleed and major bleeding, which can be catastrophic. It only takes one patient to die of a catastrophic bleed to make a physician retreat from the use of tPA. Catheter-directed ultrasound thrombolysis allows us to give one-fifth of the dose and still get the same effect.
Research has shown without a doubt that a systemic dose of tPA works. It will do the same thing that catheter-directed thrombolysis does with the Ekos catheter. The right ventricle get smaller, the right ventricular/left ventricular ratio decreases, and the patient does much better in the short term as well as the long term. But now, with the use of the Ekos catheter, only one-fifth of the dose of tPA is required. It should make physicians less reluctant to use tPA to treat these patients.
The SEATTLE II trial (A Prospective, Single-arm, Multi-center Trial of EkoSonic Endovascular System and Activase for Treatment of Acute Pulmonary Embolism) is looking at submassive and massive pulmonary embolism patients. Can you tell us about the trial?
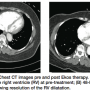
SEATTLE II began in June 2012 with the goal of 150 patients. Enrollment is going extremely well and over halfway at this point. The trial has two endpoints. First, efficacy: measuring the improvement in right ventricular/left ventricular ratio after use of the ultrasound-assisted thrombolysis catheter in submassive or massive pulmonary embolism (PE). Patients get a pre-treatment computed tomography angiography (CTA) of the chest, we perform the treatment, and then the patient undergoes a post treatment CTA of the chest 48 hours later. The second endpoint is safety: looking at the complications that may develop during treatment.
The pretreatment CTA is also used for diagnosis?
Yes, the CTA is the most frequently used imaging study to diagnose pulmonary embolism. From the CTA, we can also measure the right ventricular/left ventricular ratio; this allows us to determine eligibility for the trial.
Yet only a very small amount of PE patients are actually diagnosed.
That is correct, and it is unfortunate, because there are 600,000 cases yearly in the United States, with over 200,000 deaths due to PE. It is the most preventable cause of death for in-hospital patients. PE is a very common disease, yet it is not recognized nearly enough, and I think it is misdiagnosed frequently. I can’t tell you the number of patients I eventually get called to take care of that have had multiple trips to various emergency rooms, complaining of chest pain and shortness of breath. A CTA was not done and these patients have been signed out with any number of different diagnoses.
Is there a particular group of patients at risk of a PE?
Any patient with a diagnosis of cancer or a patient who is immobilized for any period of time is at risk for pulmonary embolism. Unprovoked PE can often be the first manifestation of an underlying hypercoagulable disorder. What’s interesting about PE is that we are not talking about the elderly who have multiple medical problems. PE can strike the very young and the very healthy. We see it all the time, the 20- or 30-year-old athlete and the 42-year-old businessman who steps off of a plane after flying cross-country. It can strike anybody.
Yet I would say as many as 75% of all physicians don’t realize that PE should be separated into three categories and are not aware of the importance of the submassive category. The patient doesn’t know how sick they are and the doctor frequently doesn’t know the seriousness of the submassive category.
There are three categories of pulmonary embolism (PE) patients:
- Massive PE patients account for 5%. These patients present in cardiogenic shock, so they are the most serious group, and they have a high mortality rate.
- The submassive group accounts for 40%. Submassive patients are hemodynamically stable, with normal blood pressure, but present with evidence of right ventricular dysfunction or right heart enlargement, so with imminent right heart failure.
- The remaining patients, about 55%, will present as minor PE patients, and we normally don’t treat them with anything other than the traditional anticoagulation.
How far out are you following patients in the SEATTLE II trial?
We see patients 30 days after discharge to make sure there have been no readmissions or any other complications that develop. I continue to follow these patients out to a year. When I was at the Veith meeting last year, we presented the results of 24 patients. My single-center experience is currently at 55 patients. We are still seeing the same degree of regression in the right ventricular/left ventricular ratio.
Current accepted standard of care in the treatment of patients with PE is anticoagulation for all three categories: massive, submassive and minor PE. When patients get to the point where they are obviously dying, the physician will usually get more aggressive and give tPA systemically, and this has saved lives. If the patients are easily resuscitated and they become more stable, the general trend is to just anticoagulate. Forty percent of all PE patients are submassive patients, which means they are hemodynamically stable. They have a normal blood pressure, normal pulse rate, and normal oxygen saturation, but their right heart is enlarged. Right heart enlargement puts patients at risk of developing sudden death, because they can easily slide into the massive PE category if they re-embolize or deteriorate. All three categories of PE patients should not be treated the same. It is evident we should be treating these patients differently and we should be more aggressive with the massive and submassive patients.
The Ekos catheter takes 20-25 minutes to put into place. It is very well tolerated. Within 12 hours for a bilateral PE and 24 hours for a unilateral PE, the treatment is over and the patient can leave the ICU. They are anticoagulated, of course, but the risk of sudden death is diminished, because that obstruction has been immediately improved. The right heart normalizes, an immediate benefit. The long-term benefit is in preventing primary pulmonary hypertension that can develop as many as ten years later, potentially turning the patient into a cardiac cripple.
We also measure pulmonary artery pressures pre and post treatment. After we treat the patient, the PA pressure can be measured through the existing Ekos catheter with the patient in the ICU. We have seen a nice reduction in pulmonary hypertension. We are looking for a decrease in the right ventricular/left ventricular ratio within 48 hours ± 6 hours, along with a safety profile.
With traditional therapy, a post treatment CTA is rarely performed. We have shown that ultrasound-assisted thrombolysis allows for a significant decrease in right ventricular/left ventricular ratio, and with that reduction, the patient benefits greatly in the both the short term and long term. I believe ultrasound-assisted thrombolysis is going to be a game-changer. Quite possibly, we are going to redefine standard of care for as many as 50% of PE patients.
Dr. Tod Engelhardt can be contacted at tengelha@ejgh.org.
Recommended reading
- Engelhardt TC. Catheter-directed ultrasound thrombolysis and the reduction of right ventricular dysfunction in acute pulmonary embolism. Cath Lab Digest 2012 Jan; 20(1). Available online at https://www.cathlabdigest.com/articles/VeithSymposium-Hot-Topics-Peripheral-Vasculature?page=3. Accessed December 12, 2012.










