Optical Coherence Tomography Findings During “Evolving” Stent Thrombosis
ABSTRACT
Stent thrombosis is a rare but feared complication. While intravascular ultrasound and optical coherence tomography are frequently used to unravel mechanical predisposing factors in patients suffering from this dreadful complication, no information exists on the early pathophysiology of stent thrombosis before coronary flow has been interrupted. We present a case where optical coherence tomography was used to gain novel insights into the earliest stages of stent thrombosis. In our patient with a possible “evolving” stent thrombosis, optical coherence tomography revealed a unique “spider web-like” or “cheese-like” thrombus morphology not previously reported.
Stent thrombosis (ST) is a rare event that is associated with high morbidity and mortality.1 While subacute ST mechanisms appear to be predominantly mechanical, late ST seems to be associated with delayed endothelialization. Angiography is unable to reveal the mechanical factors associated with the occurrence of this unique adverse event. However, intravascular ultrasound (IVUS) has been classically used to unravel the potential mechanical factors predisposing to ST. These include underexpansion, malapposition, inflow-outflow disease, and residual dissections.1 More recently, optical coherence tomography (OCT) has also been used in this setting. Given its ultra-high resolution (10–15 µm), OCT offers a unique opportunity to assess even minor underlying mechanical substrates that may play a role in the genesis of ST. Only anecdotal case reports of OCT in patients with definitive very late stent thrombosis have been reported in the literature,2,3 mostly confirming prior data from IVUS studies.
Most previous studies assessed IVUS findings immediately after stent deployment and correlated them with subsequent episodes of ST.4,5 Conversely, other investigators have used IVUS or OCT during the repeated interventions required in patients suffering from clinical episodes of ST.6,7 Nevertheless, very scarce information exists regarding the most initial phases of ST while coronary flow is not yet compromised.
We describe OCT findings in an asymptomatic patient with ongoing stent thrombosis. These unique findings complement previous knowledge and shed additional light into the pathophysiology of this feared complication.
Case Report
 A 52-year-old man with cardiac risk factors of hypertension, hyperlipidemia, obesity, and smoking suffered cardiorespiratory arrest while at the orthopedic outpatient facility. Bystanders immediately commenced cardiopulmonary resuscitation; he was intubated and had return of spontaneous circulation within 30 minutes of resuscitation. The electrocardiogram showed inferior ST-segment elevation and the patient was subsequently referred for urgent cardiac catheterization. His coronary angiogram showed 100% thrombotic occlusion of
A 52-year-old man with cardiac risk factors of hypertension, hyperlipidemia, obesity, and smoking suffered cardiorespiratory arrest while at the orthopedic outpatient facility. Bystanders immediately commenced cardiopulmonary resuscitation; he was intubated and had return of spontaneous circulation within 30 minutes of resuscitation. The electrocardiogram showed inferior ST-segment elevation and the patient was subsequently referred for urgent cardiac catheterization. His coronary angiogram showed 100% thrombotic occlusion of 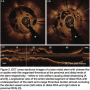 the distal right coronary artery (RCA) just before the crux and a borderline stenosis of the proximal first diagonal branch. He underwent primary intervention to the RCA with adjuvant dual oral antiplatelet therapy (loading dose of 600 mg of clopidogrel and 300 mg of aspirin) and glycoprotein IIb/IIIa inhibitors (abciximab). Subsequently an aspiration catheter (ExportAP, Medtronic Medical) was used multiple times to retrieve thrombotic material and improve coronary blood flow to distal vessel. Finally, a bare metal stent (AvantGarde, Carbostent, Sorin-Biomenco, Italy) 3.0 x 16 mm was deployed at 16 atmospheres with excellent angiographic result, confirmed in orthogonal views and TIMI III flow. A small posterolateral branch became jailed by the stent without any compromise of coronary blood flow. Patient was transferred to the intensive care unit for ongoing general management. An elective coronary angioplasty to the proximal first diagonal branch was planned, pending improvement in his neurological status.
the distal right coronary artery (RCA) just before the crux and a borderline stenosis of the proximal first diagonal branch. He underwent primary intervention to the RCA with adjuvant dual oral antiplatelet therapy (loading dose of 600 mg of clopidogrel and 300 mg of aspirin) and glycoprotein IIb/IIIa inhibitors (abciximab). Subsequently an aspiration catheter (ExportAP, Medtronic Medical) was used multiple times to retrieve thrombotic material and improve coronary blood flow to distal vessel. Finally, a bare metal stent (AvantGarde, Carbostent, Sorin-Biomenco, Italy) 3.0 x 16 mm was deployed at 16 atmospheres with excellent angiographic result, confirmed in orthogonal views and TIMI III flow. A small posterolateral branch became jailed by the stent without any compromise of coronary blood flow. Patient was transferred to the intensive care unit for ongoing general management. An elective coronary angioplasty to the proximal first diagonal branch was planned, pending improvement in his neurological status.
 Four weeks later, the patient was referred for elective coronary angioplasty to the proximal first diagonal branch. Dual antiplatelet therapy was administered initially via a nasogastric tube while intubated. He was extubated with minimal residual cognitive deficits, particularly short-term memory and orientation, and no additional signs of ischemia were reported. During the procedure, the previous proximal stenosis in the first diagonal branch was deemed mild after nitroglycerin administration. Thus, a decision was made to review the stent in
Four weeks later, the patient was referred for elective coronary angioplasty to the proximal first diagonal branch. Dual antiplatelet therapy was administered initially via a nasogastric tube while intubated. He was extubated with minimal residual cognitive deficits, particularly short-term memory and orientation, and no additional signs of ischemia were reported. During the procedure, the previous proximal stenosis in the first diagonal branch was deemed mild after nitroglycerin administration. Thus, a decision was made to review the stent in  the RCA in the same setting. Surprisingly, the stent in the RCA showed a severe, subclinical, and possibly ongoing ST with a particularly speckled appearance (Figures 1A and 1B). This image persisted despite nitroglycerin administration with coronary flow of TIMI II. Following this unexpected finding, a ChoICE PT wire (Boston Scientific, Natick, Massachusetts) was advanced across the stent into the distal posterior descending coronary artery. OCT imaging (DragonFly catheter and C7XR system, LightLab
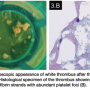
the RCA in the same setting. Surprisingly, the stent in the RCA showed a severe, subclinical, and possibly ongoing ST with a particularly speckled appearance (Figures 1A and 1B). This image persisted despite nitroglycerin administration with coronary flow of TIMI II. Following this unexpected finding, a ChoICE PT wire (Boston Scientific, Natick, Massachusetts) was advanced across the stent into the distal posterior descending coronary artery. OCT imaging (DragonFly catheter and C7XR system, LightLab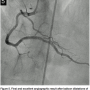 Imaging) was then performed, showing a “cheese-like” or “spider web-like” structure composed of highly reflective material throughout the stent (Figures 2A–2C). This “spider web-like” occlusive thrombus did not induce shadowing of distal structures and stent expansion could be readily identified. Moderate underexpansion (expansion of 76%) of its distal segment was recognized (measured as the ratio of minimal stent cross-sectional area divided by the mean of proximal and distal reference lumen areas). However, strut malapposition was not detected. Then, an aspiration catheter (ExportAP, Medtronic) was successfully used with improvement of coronary blood flow after retrieval of a large amount of a distinct “white” thrombotic material (Figures 3A and 3B). Finally, the stent was postdilated with a 3.5 x 14 mm Mercury balloon (Abbott Vascular, Abbott Park, Illinois) at 18 atmospheres. Postdilation, OCT showed improved expansion of the stent (stent expansion of 95%) and marked reduction of the thrombotic load, although some persistent thrombus lining apposed to the stent struts was evident (Figures 4A and 4B). An excellent angiographic result with a TIMI III coronary flow was eventually obtained, with mild transient flow deterioration of the posterolateral branch (Figure 5). Clinical course was uneventful and no rise in cardiac markers was detected.
Imaging) was then performed, showing a “cheese-like” or “spider web-like” structure composed of highly reflective material throughout the stent (Figures 2A–2C). This “spider web-like” occlusive thrombus did not induce shadowing of distal structures and stent expansion could be readily identified. Moderate underexpansion (expansion of 76%) of its distal segment was recognized (measured as the ratio of minimal stent cross-sectional area divided by the mean of proximal and distal reference lumen areas). However, strut malapposition was not detected. Then, an aspiration catheter (ExportAP, Medtronic) was successfully used with improvement of coronary blood flow after retrieval of a large amount of a distinct “white” thrombotic material (Figures 3A and 3B). Finally, the stent was postdilated with a 3.5 x 14 mm Mercury balloon (Abbott Vascular, Abbott Park, Illinois) at 18 atmospheres. Postdilation, OCT showed improved expansion of the stent (stent expansion of 95%) and marked reduction of the thrombotic load, although some persistent thrombus lining apposed to the stent struts was evident (Figures 4A and 4B). An excellent angiographic result with a TIMI III coronary flow was eventually obtained, with mild transient flow deterioration of the posterolateral branch (Figure 5). Clinical course was uneventful and no rise in cardiac markers was detected.
Discussion
Stent thrombosis is currently a rare but devastating event complicating 1% of patients undergoing coronary stenting. The pathophysiology of ST is complex and multifactorial. A great number of risk factors have been identified during the past years. In early stent thrombosis, numerous studies have reported the predominance of mechanical and anatomic etiologies underlying ST, as is the case in our patient where distal underexpansion of the bare metal stent, in the absence of IVUS guidance, was identified as a potential predisposing factor. However, it is important to mention that the thrombosis occurred in the well-expanded stent segment and not in the underexpanded distal segment. According to Virchow’s triad, vascular damage is the main factor involved in the predisposition of thrombosis and mechanical factors may play a similar role in this regard. On the other hand, from a more contemporary pathophysiologic point of view, arterial thrombosis should be considered a very complex and dynamic phenomenon where multiple factors come into play. For example, it has been demonstrated clearly that inter-individual variations in metabolism, transporters, and drug targets are important determinants of antiplatelet drug efficacy. Since 2006, various CYP mutations involving clopidogrel metabolism have been found. This condition is quite common, with carrier frequencies between 20% and 50% depending on the population and ethnicity.8
IVUS has been used to optimize stent deployment and identify factors predisposing to this complication.1,4–6 Previous studies also suggest the value of OCT to gain further insight into the underlying substrate of patients with stent thrombosis as well as providing important clues on thrombus age and composition according to its optical properties.9,10 We visualized a rare phenomenon that could correspond to very early stages of subclinical and evolving stent thrombosis in a patient who was hemodynamically stable and angina free but had an impending vessel closure on coronary angiography, or a possible occlusion-recanalization process.
This rare finding cannot be classified by the actual ARC definition since it occurred in the absence of an acute coronary syndrome. However, we have pathological evidence that suggests an in-stent thrombosis. Although we believe that these images are possibly related to an ongoing ST, distinguishing between “acute or evolving” and “subacute or evolved” thrombosis is currently impossible to define. There are some characteristics that lead us to think about the possibility of an “evolving” phenomenon:
- The angiographic “speckled” appearance (Figures 1A and 1B) that persisted after nitroglycerin administration previously reported by our group as linked to a recent event;11
- A “cheese-like” or “spider web-like” OCT appearance that could correspond to an evolving ST (Figures 2A–2C);
- The pathological confirmation of a white thrombus.
- The silent clinical and electrical course of ST.
However, we cannot exclude the possibility that our images could be due to an asymptomatic thrombosis that underwent recanalization. In fact, similar OCT images in a chronic thrombosis setting have been interpreted as evidence of thrombus recanalization.12
In our patient, OCT examination provided details on the characteristics of the evolving thrombus that showed a pattern formed by multiple tracts of low backscattering protrusions without significant dorsal shadow, suggestive of an ongoing organization of white thrombus. This was confirmed by the histopathology of the material obtained by thromboaspiration. Previous reports have emphasized the unique value of OCT to differentiate red thrombus (with shadowing of distal structures) from white thrombus (without distal shadowing).10 Furthermore, although stent expansion was adequate by angiographic criteria, clear underexpansion and asymmetry of the stent was detected by OCT. Otake et al13 previously showed that underexpansion of sirolimus-eluting stents on OCT could be an important factor in thrombus formation. In the present case, a speckled angiographic pattern and spider web-like projections of white thrombus on OCT, suggestive of either an evolving stent thrombosis or an occlusion-recanalization process, was readily identified. In our previous description of an asymptomatic patient experiencing ongoing stent thrombosis, we found a similar speckled angiographic appearance at the stent site, and a soft, hypoechogenic and obstructive thrombus on IVUS.11 At that time, however, OCT was not available and we failed to fully characterize the pattern of the evolving stent thrombosis. In our current patient, OCT enabled us to recognize a distinct thrombus with cheese-like or spider web-like appearance that suggested a unique phenomenon occurring within the stent. Accordingly, it is tempting to speculate that ST does not occur as a result of a confined, localized, and parietal thrombus lining that increases in size and eventually occludes the stent, but rather from spider web-like thrombus (accounting for the speckled angiographic pattern), initially encompassing only a segment of the stent, but allowing a TIMI III coronary flow, and eventually progressing with obliteration of residual channels and leading to vessel closure.
Conclusion
OCT may be used in selected patients with evolving and subclinical stent thrombosis to visualize different histologic and optical patterns of coronary thrombi, shed light into different predisposing factors, and guide subsequent patient management.
The authors can be contacted via Dr. Fernando Alfonso at falf@hotmail.com.
References
- Alfonso F, Suárez A, Angiolillo DJ, et al. Findings of intravascular ultrasound during acute stent thrombosis. Heart 2004;90(12):1455–1459.
- Schinkel AF, Barlis P, van Beusekom HM, Serruys PW, Regar E. Images in intervention. Optical coherence tomography findings in very late (4 years) paclitaxel-eluting stent thrombosis. JACC Cardiovasc Interv 2008;1(4):449–451.
- Jiménez-Valero S, Moreno R, Sánchez-Recalde A. Very late drug-eluting stent thrombosis related to incomplete stent endothelialization: In-vivo demonstration by optical coherence tomography. J Invasive Cardiol 2009;21(9):488–490.
- Uren NG, Schwarzacher SP, Metz JA, et al. Predictors and outcomes of stent thrombosis: An intravascular ultrasound registry. Eur Heart J 2002;23(2):124–132.
- Cheneau E, Leborgne L, Mintz GS, et al. Predictors of subacute stent thrombosis: Results of a systematic intravascular ultrasound study. Circulation 2003;108(1):43–47.
- Alfonso F, Suárez A, Pérez-Vizcayno MJ, et al. Intravascular ultrasound findings during episodes of drug-eluting stent thrombosis. J Am Coll Cardiol 2007;50(21):2095–2097.
- Cook S, Wenaweser P, Togni M, et al. Incomplete stent apposition and very late stent thrombosis after drug-eluting stent implantation. Circulation 2007;115(18):2426–2434.
- Zwart B, van Werkum JW, Heestermans AA, Ten Berg JM. Coronary stent thrombosis in the current era: Challenges and opportunities for treatment. Curr Treat Options Cardiovasc Med 2010;12(1):46–57.
- Gonzalo N, Escaned J, Alfonso F, et al. Is refined OCT guidance of stent implantation needed? Eurointervention 2010 May;6 Suppl G:G145–153.
- Kume T, Akasaka T, Kawamoto T, et al. Assessment of coronary arterial thrombus by optical coherence tomography. Am J Cardiol 2006;97(12):1713–1717.
- Alfonso F, García-Touchard A, López-Meneses M, et al. Acute stent thrombosis visualized by intravascular ultrasound. J Invasive Cardiol 2001;13(7):531–534.
- Cho JM, Raffel OC, Stone JR, Kim CJ, Jang IK. Spontaneous recanalization of a coronary artery after thrombotic occlusion: In vivo demonstration with optical coherence tomography. J Am Coll Cardiol 2010;55(12):1274.
- Otake H, Shite J, Ako J, et al. Local determinants of thrombus formation following sirolimus-eluting stents implantation assessed by optical coherence tomography. JACC Cardiovasc Interv 2009;2(5):459–466.
Reprinted with permission from the Journal of Invasive Cardiology 2011;23:E222–E225.
Disclosure: The authors have completed and returned the ICMJE Form for Disclosure of Potential Conflicts of Interest. No authors reported conflicts regarding the content herein.










