Novel Use of Intravascular Ultrasound in Percutaneous Aortic Balloon Valvuloplasty: A Case Report
Abstract
Percutaneous balloon aortic valvuloplasty has been limited, due to poor durability and high rates of recurrence and restenosis. Moreover, interpretation of valve gradients and valve areas post-valvuloplasty has been inconsistent due to variable effects on these parameters. We describe the novel use of intravascular ultrasound for measuring pre- and post-valvuloplasty aortic valve areas as a supplement to area measurements based on pressure gradients alone.
Introduction
Percutaneous balloon aortic valvuloplasty (PBAV), although successful in elderly patient populations with calcified aortic stenosis (AS), has had a limited role due to poor durability and high rates of recurrence and restenosis.1 However, the measurement of parameters of success have been variable due to poor correlation and interpretation of valve gradients and valve area post-valvuloplasty; although gradients may be reduced, the effect of valve area is generally small.2 We describe the novel use of intravascular ultrasound (IVUS) for measuring pre- and post-valvuloplasty aortic valve areas as a readily available supplement to area measurements based on pressure gradients alone.
Case
An 83-year-old female with prior bioprosthetic mitral valve replacement and coronary artery bypass grafting with single saphenous vein graft to the right coronary artery presented with worsening shortness of breath and fatigue. Physical examination was remarkable for a frail elderly female, with a light pallor, irregularly irregular cardiac rhythm with a grade 3/6 late-peaking systolic murmur at the right upper sternal border radiating to the carotids, and decreased breath sounds bilaterally with basilar rales. Laboratory analysis was only significant for anemia (hemoglobin 8.3 gm/dL). Electrocardiogram showed normal sinus rhythm with nonspecific ST-segment changes. Transthoracic echocardiogram revealed preserved left ventricular function with a calcified stenotic aortic valve (peak gradient of 46 mm Hg, mean gradient of 22 mm Hg, calculated valve area of 0.8 cm2), and a bioprosthetic mitral valve with mild stenosis.
 Based on findings from the transthoracic echocardiogram, diagnostic right and left heart catheterization with coronary angiography was performed for further evaluation of the aortic valve, with plans for PBAV as a bridge for further workup of anemia in the setting of decompensated heart failure and possible gastrointestinal bleeding precluding surgery and anticoagulation. Coronary angiography revealed a patent saphenous vein graft to the right coronary artery and no other angiographically significant obstructive disease. Right heart catheterization revealed mildly elevated right-sided pressures with mild stenosis of the mitral valve prosthesis (peak gradient of 10 mm Hg, mean gradient of 5 mm Hg, calculated valve area of 1.60 cm2). Simultaneous left ventricular and aortic pressure recordings were performed (Figure 1: peak gradient of 56 mm Hg, mean gradient of 45 mm Hg, calculated valve area of 0.53 cm2) which confirmed a severely stenotic aortic valve. The aortic valve systolic orifice area was then measured using IVUS pullback (Atlantis Pro, Boston Scientific), which reaffirmed severe AS (Figure 1: valve orifice area of 0.49 cm2).
Based on findings from the transthoracic echocardiogram, diagnostic right and left heart catheterization with coronary angiography was performed for further evaluation of the aortic valve, with plans for PBAV as a bridge for further workup of anemia in the setting of decompensated heart failure and possible gastrointestinal bleeding precluding surgery and anticoagulation. Coronary angiography revealed a patent saphenous vein graft to the right coronary artery and no other angiographically significant obstructive disease. Right heart catheterization revealed mildly elevated right-sided pressures with mild stenosis of the mitral valve prosthesis (peak gradient of 10 mm Hg, mean gradient of 5 mm Hg, calculated valve area of 1.60 cm2). Simultaneous left ventricular and aortic pressure recordings were performed (Figure 1: peak gradient of 56 mm Hg, mean gradient of 45 mm Hg, calculated valve area of 0.53 cm2) which confirmed a severely stenotic aortic valve. The aortic valve systolic orifice area was then measured using IVUS pullback (Atlantis Pro, Boston Scientific), which reaffirmed severe AS (Figure 1: valve orifice area of 0.49 cm2).
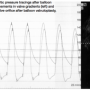 Due to the patient’s multiple co-morbidities and poor candidacy for immediate surgery, PBAV was pursued as a bridge for palliation while undergoing further workup for anemia. Using standard PBAV technique during rapid right ventricular pacing at 180 beats per minute, a Tyshak II 20 x 60 mm balloon (NuMed, Inc.) was inflated for balloon valvuloplasty, with 2 inflations after a 5-minute interval. Repeat left ventricular and aortic pressure recordings revealed significant improvement in aortic valve gradients post-valvuloplasty (Figure 2: peak gradient of 20 mm Hg, mean gradient of 16 mm Hg, calculated valve area of 0.90 cm2). The aortic valve orifice area was again measured using IVUS pullback, which was consistent with the improvement in calculated valve area (Figure 2: valve orifice area of 1.07 cm2).
Due to the patient’s multiple co-morbidities and poor candidacy for immediate surgery, PBAV was pursued as a bridge for palliation while undergoing further workup for anemia. Using standard PBAV technique during rapid right ventricular pacing at 180 beats per minute, a Tyshak II 20 x 60 mm balloon (NuMed, Inc.) was inflated for balloon valvuloplasty, with 2 inflations after a 5-minute interval. Repeat left ventricular and aortic pressure recordings revealed significant improvement in aortic valve gradients post-valvuloplasty (Figure 2: peak gradient of 20 mm Hg, mean gradient of 16 mm Hg, calculated valve area of 0.90 cm2). The aortic valve orifice area was again measured using IVUS pullback, which was consistent with the improvement in calculated valve area (Figure 2: valve orifice area of 1.07 cm2).
Discussion
PBAV is primarily used as a palliative therapeutic option for relief of severe AS in patients who are poor candidates for definitive surgical therapy. The rationale for limited use of PBAV in severe AS is due to poor durability and high rates of recurrence.1 Some studies have even suggested lower survival at follow-up compared to surgical valve replacement.3 However, PBAV has been supported by a Class IIb recommendation in the current guidelines as a bridge to surgery in patients with AS who are at high risk for surgery.4 Furthermore, with the advent of transcatheter aortic valve implantation (TAVI), PBAV has been recently described as a feasible and safe approach as a bridge to high-risk TAVI.
PBAV, although transient, provides a relatively modest improvement in valve function and a degree of functional improvement.3 Parameters of success, however, have been variable, due to poor correlation and interpretation of valve gradients and valve area post-valvuloplasty. Although gradients may be reduced, the effect of valve area is generally small.2 Therefore, alternative methods of obtaining measurements of aortic valves pre- and post-valvuloplasty are warranted.
Since the initial clinical experience with IVUS, this technique has gained acceptance as both a research and clinical tool for supplementation of findings when conventional methods provide unclear or disparate results in the evaluation of coronary atherosclerosis. We demonstrate that by using a peripheral IVUS catheter with adequate beam penetration, accurate aortic valve orifice area can be obtained readily both pre- and post-valvuloplasty as additional parameters for assessment of AS.
Dr. George can be contacted at jcgeorgemd@hotmail.com.
Disclosure: Dr. George reports he is a consultant for Boston Scientific Corporation. Dr. Shamshad reports no conflicts of interest regarding the content herein.
References
- McKay RG, Safian RD, Lock JE, et al. Balloon dilatation of calcific aortic stenosis in elderly patients: post-mortem, intra-operative and percutaneous valvuloplasty studies. Circulation 1986;74:119-125.
- Deligonul U, Kern MJ. Interpretation of cardiac pathophysiology from pressure waveform analysis: percutaneous balloon valvuloplasty. Cathet Cardiovasc Diagn 1991;24(2):111-120.
- Otto CM, Mickel MC, Kennedy JW, et al. Three-year outcome after balloon aortic valvuloplasty. Insights into prognosis of valvular aortic stenosis. Circulation 1994;89:642-650.
- 2008 Focused Update incorporated into the ACC/AHA 2006 Guidelines for the management of patients with valvular heart disease. J Am Coll Cardiol 2008;52:e1-e142.










