Minimizing Pain During Cath Lab Procedures
Introduction
 The focus of this article is to address patient comfort when local anesthetic is used to numb procedure sites during procedures in the cardiac catheterization laboratory (CCL). The Joint Commission, Press-Ganey, and the Hospital Consumer Assessment of Healthcare Providers and Systems (HCAPS) indicate pain management and patient satisfaction as measures of quality healthcare and patient satisfaction which will be tied to federal reimbursement in the future.1 HCAPS surveys are completed by patients either online or through the mail. Three questions regarding pain management directly correlate to the quality of a patient’s hospital experience. They are:
The focus of this article is to address patient comfort when local anesthetic is used to numb procedure sites during procedures in the cardiac catheterization laboratory (CCL). The Joint Commission, Press-Ganey, and the Hospital Consumer Assessment of Healthcare Providers and Systems (HCAPS) indicate pain management and patient satisfaction as measures of quality healthcare and patient satisfaction which will be tied to federal reimbursement in the future.1 HCAPS surveys are completed by patients either online or through the mail. Three questions regarding pain management directly correlate to the quality of a patient’s hospital experience. They are:
a) “During this hospital stay, did you need medicine for pain?”
b) “During this hospital stay, how often was your pain well controlled?”
c) “During this hospital stay, how often did hospital staff do everything they could to help you with your pain?”1
While accreditation issues are important, the most important reasons for pain management are ethical. Patient comfort and pain relief should be one of the top concerns of caregivers. There is pain associated with many routine hospital procedures that can be minimized by bedside caregivers implementing evidence-based practices. Lidocaine is the most commonly used local anesthetic in the CCL. Lidocaine is an acidic solution that causes a stinging and burning sensation that is unpleasant for patients. The question, “Why does this solution have to sting and burn?” or “Why does this have to be the worst part of the procedure?” begs an answer.
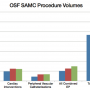
OSF Saint Anthony Medical Center (SAMC) is 254-bed tertiary care facility located on a 100-acre campus near Interstate 90 and US Business 20 in Rockford, Illinois. SAMC has achieved Magnet status and places a high importance upon evidence-based practice. The cardiac catheterization laboratory (CCL) has evolved into a very busy and cutting-edge endovascular lab performing diagnostic and interventional procedures on not only coronary arteries, but also carotids, renals, mesenteric, and other peripheral vasculature. The Saint Anthony CCL team includes 14 cardiologists, 12 nurses, 17 radiology technologists, 7 radiologists, and 2 cardiovascular surgeons. Routine and emergent procedures are performed daily, and Figure 1 displays our procedure volumes.
The pace and redundancy of work in the CCL can cause the team to lose touch with the patient’s perspective. The CCL team must remember that what seems routine is not routine for the patient or their families. There is a tendency to mentally minimize the pain inflicted upon patients by physicians, nurses and technologists; this is particularly true of the pain experienced during routine procedures.
The future of healthcare and medical reimbursement nationwide has caused many hospitals to undergo major restructuring with a goal of minimizing cost. It is important to also minimize the risk of dehumanizing patient care in the process. Caring must remain at the core of our clinical practice.
Jean Watson is a prominent nurse theorist who created her Theory of Human Caring in 1988 while on faculty at the University of Colorado. SAMC has chosen Watson’s model of caring to base nursing practice upon, because it is closely linked to the mission of OSF SAMC. Jean Watson states in her theory of Caring, “We must cultivate sensitivity to one’s self and to others.”2
CCL procedures can be emotionally terrifying, embarrassing, and physically painful. Patients entrust their body into our care. It is good practice to put oneself in the patient’s position and ask, “How would I like to be treated?” Patients are disrobed, brought into cold rooms, and placed on hard, narrow tables. Upon starting the procedure, the physician frequently states, “This is the worst part of the procedure, this will sting and burn,” as lidocaine is injected into the procedure site, usually the groin, wrist or chest. Lessening the sting and burn of the solution being injected appeared to be an important goal in making these procedures more comfortable for the patients.
Project launch
This project consisted of a literature review, a Magnet query, a test of change, communication of results, and finally, a plan to hardwire the change of practice into daily routine. The Magnet query consisted of emailing other Magnet facilities’ CCLs to ask what type of solution they were using as their local anesthetic. Responses were received from six other Magnet facilities indicating that the responding Magnet facilities were using lidocaine that was not buffered and not warmed. For the purpose of this article, the term plain lidocaine will be used to refer to lidocaine that is not buffered and not warmed.
A literature review comparing different solutions and patients’ self-report of pain found that warming and buffering lidocaine has a synergistic effect in reducing the pain of local anesthetic. The conclusions of these studies were:
a) “Skin infiltration with warm buffered lidocaine is significantly less painful than infiltration with room-temperature unbuffered lidocaine”3;
b) “Buffered lidocaine without epinephrine is less painful to inject than plain lidocaine without epinephrine warmed to 102 degrees F”4;
c) “The combination of warming and buffering lidocaine had a greater effect on the reduction of infiltration pain than either warming or buffering alone”5;
d) “Results show that both buffered and warmed bupivacaine significantly reduced pain on infiltration…in addition, subjects who received warmed bupivacaine had a more rapid onset of intradermal anesthesia”6.
Howard Weiss, MD, Medical Director of Pain Management Services at SAMC, offered his expert opinion: “The use of warmed, buffered lidocaine as a local anesthetic has been shown to reduce discomfort versus plain lidocaine, without increasing the risk to the patient and should be recommended to be used in any locale where awake patients are given a local anesthetic for a procedure.”
Purpose of project
The purpose of this investigation was to determine if warming and buffering lidocaine produced less measurable pain than plain lidocaine. After a review of the literature and seeking expert opinion, a test of change comparing the two solutions was conducted. An interdisciplinary team assisted in the development and implementation of this test of change. The team consisted of two nurses, two radiology technologists, and a physician champion.
The purpose of the test of change was to validate the conclusions found in the literature review in the CCL setting. The test of change compared pain level of patients receiving plain lidocaine with pain levels of patients receiving buffered, warmed lidocaine. The patient’s self-report of pain was the tool used to measure pain experienced during the injection of the two different solutions. Consistent scripting by physicians and nurses with each patient was incorporated. This was a single-blind study with the physician and nurse knowing the type of solution and the patient unaware of the type of solution.
Intervention
 During June 2009, a convenience sample of 120 male and female adult patients was asked to rate the pain they experienced when the physician injected lidocaine into their procedure site, usually the groin. The patients had the cognitive ability to comprehend and use the 0-10 pain scale. Half of the patients received plain lidocaine and the other half received buffered, warmed lidocaine. Ninety-two percent of the patients receiving warmed, buffered lidocaine rated the discomfort of the lidocaine infiltration as a 0 on the 0-10 pain scale. Sixty-two percent of the group receiving plain lidocaine had self-reported pain scale ratings of moderate to severe intensity (3-8) on the 0-10 pain scale. The SAMC CCL has now implemented the change to warmed, buffered lidocaine as the preferred solution for local anesthetic and hopes to extend this change of practice throughout the hospital. This change of practice is in addition to most patients receiving intravenous conscious sedation, usually hydromorhone or sublimaze with midazolam.
During June 2009, a convenience sample of 120 male and female adult patients was asked to rate the pain they experienced when the physician injected lidocaine into their procedure site, usually the groin. The patients had the cognitive ability to comprehend and use the 0-10 pain scale. Half of the patients received plain lidocaine and the other half received buffered, warmed lidocaine. Ninety-two percent of the patients receiving warmed, buffered lidocaine rated the discomfort of the lidocaine infiltration as a 0 on the 0-10 pain scale. Sixty-two percent of the group receiving plain lidocaine had self-reported pain scale ratings of moderate to severe intensity (3-8) on the 0-10 pain scale. The SAMC CCL has now implemented the change to warmed, buffered lidocaine as the preferred solution for local anesthetic and hopes to extend this change of practice throughout the hospital. This change of practice is in addition to most patients receiving intravenous conscious sedation, usually hydromorhone or sublimaze with midazolam.
With the collaboration of pharmacists, pharmacy technicians, physician champion, Dr. Howard Weiss, and staff, the routine use of warmed, buffered lidocaine has become hardwired into CCL procedures. Pharmacy is currently purchasing the buffered lidocaine from a compounding pharmacy and stocks the medication stations daily. Procedure room nurses remove the number of vials or syringes needed for the day’s case load, times and dates each one with an expiration time of 24 hours, then places them into our warmers (usually around 93 degrees Fahrenheit). During the time-out prior to the start of the procedure, the warmed, buffered lidocaine is poured onto the scrub table. Utilizing this time is beneficial because it ensures that it will be warm when injected into the patient.
Discussion
Establishing the change of practice required an interdisciplinary approach, with ongoing communication of the goal and the benefit to the patients. Resistance to change was encountered by the CCL staff and physicians. Many of the scrub technologists had a difficult time waiting for the lidocaine to be placed on the table during the time-out. The project leaders needed patience and tenacity to support and enact this change. Administration and physician support was paramount in successfully changing practice. Physicians now want to use warmed, buffered lidocaine with all their procedures. Some physicians who practice at other locations are asking those labs to change to warmed, buffered lidocaine. Dr. Eugene Silva is both an electrophysiologist and interventional cardiologist who performs CCL procedures at SAMC and another local hospital that still uses plain lidocaine. He states that he sees a night-and-day difference in the way patients react when the two different solutions are injected. The following is his statement on this issue:
“In my experience with using warmed, buffered lidocaine in one institution where I work and another where they have not made the commitment to use it, my patient’s reaction to the warmed, buffered product is that it is definitely more comfortable for the patient. I believe this helps reduce patient anxiety to the procedure. This is in both interventional cases and my device implants.”
Conclusion
Warmed, buffered lidocaine during all procedures that involve awake patients in the CCL and angiography suites has become the standard of care at OSF SAMC. Patient self-report of pain is also used, with the majority of our patients stating that they had either 0/10 pain with solution infiltration or mild discomfort. The use of warmed, buffered lidocaine as a local anesthetic has been shown to reduce discomfort versus plain lidocaine.
These results have generalizability in the CCL patient population. The practice change has replaced what was termed the most unpleasant part of the procedure for the patient with either mild or no discomfort. Focusing on improving pain control measures for this patient population has been beneficial not only for patients, but for practitioners, as we strive to alleviate as much pain and discomfort for our patients as possible and become more aware of how our action affect our patients. n
The author may be contacted at patricia.sterett@osfhealthcare.org.
This article received double-blind peer review from members of the Cath Lab Digest Editorial Board.
References
1. HCAHPS Survey Instruments. Available online at https://www.hcahpsonline.org/surveyinstrument.aspx. Accessed October 1, 2012.
2. Watson J. Nursing: Human science and human: A theory of nursing. New York: National League for Nursing; 1988.
3. Mader TJ, Playe SJ, Garb JL. Reducing the pain of local anesthetic infiltration: warming and buffering have a synergistic effect. Ann Emerg Med. 1994 Mar;23(3):550-554.
4. Bartfield JM, Crisafulli KM, Raccio-Robak N, Salluzzo RF. The effects of warming and buffering on pain of infiltration of lidocaine. Acad Emerg Med. 1995 Apr;2(4):254-258.
5. Colaric KB, Overton DT, Moore K. Pain reduction in lidocaine administration through buffering and warming. Am J Emerg Med. 1998 Jul;16(4):353-356.
6. Jones JS, Plzak C, Wynn BN, Martin S. Effect of temperature and pH adjustment of bupivacaine for intradermal anesthesia. Am J Emerg Med. 1998 Mar;16(2):117-120.










