ADVERTISEMENT
In-Hospital Management of Left Main Artery Vasospasm Following a Viral Illness
Introduction
Acute left main stenosis carries a high mortality rate. It can be due either to an atherothrombotic lesion or a localized vasospasm. We present the case of an acute left main artery vasospasm that occurred following a viral illness and that resolved after administration of intracoronary nitroglycerin. Vasospasm should be thought of in the setting of acute coronary syndrome due to stenosis of the left main artery in order to avoid unnecessary bypass.
Case presentation
An 80-year-old ex-smoker male presented to our emergency department with a four-day history of a high-grade fever, dry cough, generalized weakness, loss of appetite, lightheadedness and headache. On the day of presentation, he was very weak, dyspneic, and afebrile, only complaining of mild atypical diffuse chest pain.
On physical examination, the patient appeared ill and in moderate distress. He was found to be hypotensive with a systolic blood pressure of 76 mmHg and tachypneic with a weak pulse at 64 beats per minute. There was mild distention in the neck veins. Examination of the heart showed irregular and distant S1 and S2 with no murmurs. Basal rales were heard throughout in both lungs. His abdomen was soft with no tenderness or palpable masses. His peripheral pulses were weak. Past medical history was significant for hypertension, asbestosis, benign prostatic hypertrophy, recurrent kidney stones, and chronic kidney disease. The patient was not taking any over-the-counter medications.
His initial electrocardiogram (ECG) showed an atrial fibrillation rhythm at 62 beats per minute,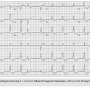 intraventricular conduction block, and diffuse 1 mm ST-segment depression and isolated 2 mm ST-elevation in aVR (Figure 1). Subsequently, the patient developed ventricular fibrillation and asystole. After rescucitation, he was emergently transferred for primary percutaneous angioplasty while his blood pressure was stabilized with neosynephrine and dopamine infusion drips. Intravenous heparin and appropriate dual antiplatelet therapy were also given.
intraventricular conduction block, and diffuse 1 mm ST-segment depression and isolated 2 mm ST-elevation in aVR (Figure 1). Subsequently, the patient developed ventricular fibrillation and asystole. After rescucitation, he was emergently transferred for primary percutaneous angioplasty while his blood pressure was stabilized with neosynephrine and dopamine infusion drips. Intravenous heparin and appropriate dual antiplatelet therapy were also given.
The cardiac enzyme profile showed elevation of creatine phosphokinase (CK) = 1026 U/L, CK-MB = 59 µg/L, and troponin I level = 23.3 ng/ml. Other laboratory tests revealed a brain natriuretic peptide of 968 pg/ml, serum creatinine of 2.37 mg/dl, and mildly elevated transaminases. The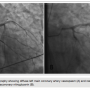 remaining blood tests were within normal limits, including white blood cell count and blood cultures. A portable chest x-ray showed multiple chronic pleural plaques.
remaining blood tests were within normal limits, including white blood cell count and blood cultures. A portable chest x-ray showed multiple chronic pleural plaques.
Coronary angiography showed a 70% left main stenosis with TIMI-2 flow down the left system and non-obstructive coronary artery disease in the remaining coronary arteries (Figure 2A). Catheter-induced spasm was less likely, though not impossible. There was no ostial dampening noted on engaging the left main. The 6 French catheter was replaced with a 5 French catheter, but the stenosis persisted. A vasospasm was suspected. The neosynephrine infusion was stopped and followed by administration of 100 µg of intracoronary nitrates. The restoration of blood flow without residual stenosis confirmed the suspected diagnosis (Figure 2B). A bedside echocardiography showed an ejection fraction of 35 to 40%, basal septal and lateral left ventricular wall hypokinesia, severe mitral regurgitation, and moderate tricuspid regurgitation.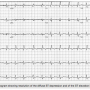
Post procedure, the patient gradually improved and the ST segment changes completely resolved on the ECG (Figure 3), while the cardiac enzymes trended down towards the normal range.
Discussion
Coronary artery spasm is an infrequent, but important, cause of acute coronary syndrome (ACS). Its incidence among patients undergoing diagnostic coronary angiography for suspected ACS is 3-4%.1,2 Vasospasm of the left main artery may be spontaneous due to inherent endothelial dysfunction (as an uncommon cause of Prinzmetal’s variant angina) or iatrogenic (catheter- or guidewire-induced). Coronary vasospasm can also be due to other factors: chemical, such as exposure to acetylcholine, cocaine, noradrenaline, substances induced by hyperventilation, or anesthetics; and physical, such as cold exposure and exercise.3 Left main coronary artery (LMCA) vasospasm can present as an ACS that causes severe hemodynamic instability and imposes challenges for management. In consequence, LMCA vasospasm should be considered in the differential diagnosis of an angiographically isolated lesion of the left main artery to avoid misdiagnosis of an atherothrombotic event.
In our case, the presence of normal coronary arteries with the exception of the left main stenosis indicated the high probability that this appearance was due to vasospasm. Prolonged coronary spasm may induce endothelial damage, release of vasoactive substances, and platelet aggregation, resulting in local thrombus formation.4 The etiology of the spasm was unclear, but it was unlikely that it was due to the mismatch between the artery and the catheter size, since the vasospasm persisted despite the use of a smaller, 5 French catheter. In addition, a catheter-induced spasm usually appears in cases of size mismatch in an angulated artery, and is seen within 1 mm of the catheter tip, in contrast to the current situation, in which the spasm was diffuse, irregular and eccentric.
The second step was to withhold any offending agents that could precipitate spasm, such as neosynephrine and dopamine. Neosynephrine is a potent and direct-acting alpha-adrenergic agonist with virtually no beta-adrenergic activity; it produces systemic arterial vasoconstriction, causing an increase in the mean arterial pressure and systemic vascular resistance without affecting cardiac output. Dopamine, usually in doses of 5 to 10 mcg/kg per minute, stimulates beta-1 adrenergic receptors and increases cardiac output, predominantly by increasing stroke volume with variable effects on heart rate; it can have some mild alpha-adrenergic receptor activation that also increases systemic vascular resistance.
In this case, spasm was persistent despite the measures undertaken; therefore, the final step was to inject intracoronary nitroglycerin that dilated the spastic artery, leading to resolution of the ST segment changes, improvement of the left ventricular function, and the blood pressure.
The etiology of the spasm could be multifactorial. In our case, we assumed that the intravenous neosynephrine infusion was a contributing factor to this spontaneously occurring LMCA vasospasm. In addition, the patient presented few days after a viral prodromal illness. The viral etiology of spasm could not be excluded; it could be isolated or as part of a myocardial inflammatory process.5 Acute myocarditis, especially of infectious origin (Coxsackie virus), may present as acute myocardial infarction (AMI) in patients with normal coronary arteries.4,6 The exact pathogenesis linking the viral infection with the onset of AMI remains unclear. Viremia may induce platelet alteration, resulting in agglutination and lysis with release of vasospastic substances (e.g., thromboxane A2) and final formation of coronary thrombosis.7 A study done by Klein et al8 proposed an association between myocarditis and the endothelial dysfunction of the epicardial coronary arteries. The infection could produce inflammation of the endothelium, resulting in loss of the endothelium-mediated vasodilatation and causing myocardial ischemia. A deficiency in endothelial NO activity in spasm arteries has been shown to play also a role in the mechanism of coronary spasm.9
Multiple studies have assessed the relationship between the ST-segment elevation in aVR and either the acute obstruction in the LMCA or the presence of triple-vessel disease. It is important to recognize such correlation in the setting of malignant ventricular fibrillation and acute coronary syndrome.1,10
The left main vasospasm can be considered as a variant of Prinzmetal’s angina in which a coronary angiography will show normal arteries or nonobstructive plaques in the presence of what appears to be a left main stenotic lesion; in such cases, care should be undertaken not to directly refer these patients to bypass surgery, as the resolution of the spasm would lead to the occlusion of the internal mammary.6
It is important to check for the catheter size, the presence of waveform dampening and the administration of intracoronary nitroglycerin after withholding vasospastic agents. However, in the setting of recurrent episodes of idiopathic vasospasm, few articles have reported revascularization as the ultimate treatment of choice either with percutaneous angioplasty or surgical bypass, in case the combined medical use of nitrates and calcium channel blockers fails to prevent further acute events.11
Conclusion
The recognition of the LMCA vasospasm is crucial to avoid unnecessary bypass surgery. It should be suspected in the setting of an isolated lesion in the left main artery. The coexistence of intravenous vasoconstrictors could exacerbate such a spasm. LMCA vasospasm should always be considered in the setting of an acute coronary syndrome with the ST segment elevation in aVR.
This article received a double-blind peer review from members of the Cath Lab Digest Editorial Board.
The authors may be contacted via Hassan Baydoun, MD, at baydounhassan@hotmail.com.
References
- Kosuge M, Kimura K, Ishikawa T, Ebina T, Shimizu T, Hibi K, et al. Predictors of left main or three-vessel disease in patients who have acute coronary syndromes with non-ST-segment elevation. Am J Cardiol. 2005 Jun 1; 95(11): 1366-1369.
- Duygu H, Yavuzgil O, Erturk U, Zoghi M, Ozerkan F. ST-segment elevation in lead augmented vector right may also be caused by diffuse left main coronary artery vasospasm without fixed stenosis. Clin Cardiol. 2008 Apr; 31(4): 179-182. doi: 10.1002/clc.20166.
- González Enríquez S, de la Torre Hernández JM, Sainz Laso F. [Clinical episode of suggestive left main coronary artery spasm after gingival anaesthetic infiltration]. Rev Esp Cardiol. 2003 Oct; 56(10): 1033-1034.
- Sztajzel J, Mach F, Righetti A. Role of the vascular endothelium in patients with angina pectoris or acute myocardial infarction with normal coronary arteries. Postgrad Med J. 2000 Jan; 76(891): 16-21.
- Iwasaki K, Kusachi S, Tominaga Y, Kita T, Taniguchi G. Coronary artery spasm demonstrated by coronary angiography in a patient with acute myocarditis resembling acute myocardial infarction; a case report. Jpn J Med. 1991 Nov-Dec; 30(6): 573-577.
- Ferguson DW, Farwell AP, Bradley WA, Rollings RC. Coronary artery vasospasm complicating acute myocarditis. A rare association. West J Med. 1988 Jun; 148(6): 664-669.
- Spodick DH. Infection and infarction. Acute viral (and other) infection in the onset, pathogenesis, and mimicry of acute myocardial infarction. Am J Med. 1986 Oct; 81(4): 661-668.
- Klein RM, Schwartzkopff B, Strauer BE. Evidence of endothelial dysfunction of epicardial coronary arteries in patients with immunohistochemically proven myocarditis. Am Heart J. 1998 Sep; 136(3): 389-397.
- Kugiyama K, Yasue H, Okumura K, Ogawa H, Fujimoto K, Nakao K, et al. Nitric oxide activity is deficient in spasm arteries of patients with coronary spastic angina. Circulation. 1996 Aug 1; 94(3): 266-271.
- Yamaji H, Iwasaki K, Kusachi S, Murakami T, Hirami R, Hamamoto H, et al. Prediction of acute left main coronary artery obstruction by 12-lead electrocardiography. ST segment elevation in lead aVR with less ST segment elevation in lead V(1). J Am Coll Cardiol. 2001 Nov 1; 38(5): 1348-1354.
- Chou H, Lim K, Ko Y. Treatment of spontaneous left main coronary artery spasm with a drug-eluting stent. Acta Cardiol Sin. 2009; 25: 43-46.
1Department of Internal Medicine, Staten Island University Hospital, Staten Island, New York; 2Department of Cardiology, Staten Island University Hospital, Staten Island, New York










