With the Goal of Predicting STEMIs, Studying Lipid Core Plaque Defined by Near-Infrared Spectroscopy
Dr. Madder discusses a study evaluating lipid core plaque presence and amount at the culprit lesion site of ST-segment elevation myocardial infarction (STEMI) patients.1 A combination near-infrared spectroscopy (NIRS)-intravascular ultrasound (IVUS) catheter (Infraredx, Inc.) was used to image the coronary artery. NIRS has a proven ability2 to identify and quantify lipid core plaque.
lesion site of ST-segment elevation myocardial infarction (STEMI) patients.1 A combination near-infrared spectroscopy (NIRS)-intravascular ultrasound (IVUS) catheter (Infraredx, Inc.) was used to image the coronary artery. NIRS has a proven ability2 to identify and quantify lipid core plaque.
Your study sought to identify whether the presence and amount of lipid core plaque might have predictive value for STEMIs.
In the United States alone, there are roughly one million myocardial infarctions (MIs) per year. Of these events, we still cannot predict even a single MI before it occurs. Roughly one-third of MIs are STEMIs and they represent a major public health problem. The impetus for our study is the need to develop a scientific method through which we can perhaps one day predict a STEMI before it actually occurs.
The trial began in January 2012 and enrolled 20 acute STEMI patients undergoing primary percutaneous coronary intervention. The vessel was opened using either aspiration thrombectomy or an undersized balloon, and once TIMI-3 flow was established, the combined NIRS-IVUS catheter was used to image the culprit vessel. We then compared the STEMI culprit lesion characteristics to non-culprit sites in the artery, and then also to autopsy controls.
What information is available via the NIRS-IVUS imaging catheter?
This is a single catheter that combines NIRS and IVUS, thereby providing both NIRS and IVUS imaging with a single pullback within the artery. The IVUS images are helpful to assess certain vessel characteristics during PCI, including plaque burden, percent stenosis, lesion length, and reference vessel size. The NIRS images demonstrate whether lipid core plaque is present within the artery and can also provide information regarding the quantity of lipid present. Imaging with NIRS produces a ‘chemogram’, which represents a map of the artery. The chemogram is oriented in such a way that the right side of the image represents the distal aspect of the artery and the left side represents the proximal aspect of the artery. The x-axis is positioned in millimeters along the artery and the y-axis is rotation around the vessel in degrees. Red on the chemogram indicates the absence of lipid and yellow indicates the presence of lipid at any site.
One of the advantages of NIRS over both optical coherence tomography (OCT) and IVUS with respect to plaque characterization is the fact that NIRS provides a fully automated assessment of lipid content within the vessel in a quick fashion. The chemogram is available within seconds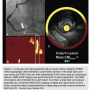 after the pullback is complete and it is incredibly easy to interpret. You simply look for yellow. If yellow is present, the NIRS system can rapidly provide an estimate of the quantity of lipid.
after the pullback is complete and it is incredibly easy to interpret. You simply look for yellow. If yellow is present, the NIRS system can rapidly provide an estimate of the quantity of lipid.
What did you find in your study?
We found a NIRS signature of STEMI culprit lesions, characterized by a large lipid core that often wraps circumferentially around the vessel at the culprit site. This NIRS signature is both sensitive and specific for the culprit site when compared to the non-culprit sites within the artery, and when compared to autopsy specimens. Unlike the thrombus that forms in the artery — which likely occurs in temporal proximity to STEMI onset — the lipid is likely present in the arterial wall well before the event. Because the lipid is likely present in the artery prior to STEMI onset, the NIRS signature may be detectable before the event, and could therefore be used for predicting a pending MI and possibly for prevention.
So we can possibly identify plaque that may rupture and cause a STEMI, but we’re not quite sure what to do with that information just yet.
This study does not address how the NIRS signature of STEMI culprit lesions can be used clinically at the present time. If NIRS identifies a large lipid core at a non-culprit site, most providers would probably agree that aggressive medical therapy makes sense, especially since aggressive medical therapy after an acute coronary event is beneficial anyway. At the present time, stenting a large lipid core plaque that is non-flow limiting is not acceptable in contemporary clinical practice and would be considered inappropriate.
Do you have any follow up studies planned to further investigate NIRS findings in STEMI patients?
The next step is to validate our findings in these initial 20 patients with a larger multicenter study. Such a validation study is well underway. We partnered with Dr. David Erlinge at Lund University in Sweden, and between Spectrum Health and Lund University, we have performed NIRS-IVUS imaging in more than 80 additional STEMI cases. The data for this validation study are now being analyzed. The analysis in both the initial 20-patient study and in the larger validation study is being performed by Dr. Stephen Nicholls at the South Australian Health and Medical Research Institute in Adelaide, Australia. This analysis is nearly complete.
Can we officially call lipid core plaque “vulnerable plaque”?
I don’t know that we can necessarily call it vulnerable yet. It may be a subtle nuance, but the term ‘vulnerable’ should be avoided until we have proven through additional studies that lipid core plaque detected by NIRS at non-culprit sites eventually results in a future acute coronary syndrome.
Although we can’t tell from the present study whether the NIRS signature of lipid core plaque was there before the STEMI, we suspect the lipid signal was present in the artery well before the STEMI occurred. Studies are being planned to image non-culprit arteries with NIRS in order to identify non-culprit lipid core plaques, and to follow these plaques over time to see if such lesions trigger future acute coronary events.
Was the amount of lipid core plaque at the culprit site consistent?
On average in the STEMI cases, we saw about 6-fold greater lipid in the culprit segment compared to non-culprit segments in the artery. When we compared the culprit segments to autopsy controls, there was an 87-fold increase in the lipid in the STEMI culprit site compared to autopsy control segments. STEMI culprit sites are characterized by a very large amount of lipid that has deposited at that site over time.
We identified a NIRS threshold that best identifies the STEMI culprit site: a maximum lipid core burden index (LCBI) in any 4mm segment within the culprit segment that was >400. With respect to the maximum LCBI, the highest possible value is 1000, which essentially means that the lipid core wraps around the vessel 100% of the circumference or all 360 degrees. As a crude estimate, a max LCBI of 400 represents a lipid core that wraps around the vessel, on average, about 40% of the circumference.
The maximum LCBI is the variable we tested in the ROC analysis and we found that the maximum LCBI performs extremely well in identifying the culprit segment. By ROC analysis, maximum LCBI identified the culprit segment with an area under the curve of 0.90 against a background of non-culprit segments and with an area under the curve of 0.98 against a background of autopsy controls.
What is the NIRS-IVUS catheter impact on workflow?
Any lab that is currently using IVUS can easily adopt NIRS-IVUS. The preparation and set up of the NIRS-IVUS instrument is very similar to traditional IVUS and takes only moments to complete. The pullback time for combined NIRS-IVUS is similar to a traditional IVUS run. We have found NIRS-IVUS can be performed efficiently without adversely impacting workflow.
How does NIRS compare to OCT?
OCT and NIRS often provide complementary information. The major strength of OCT is its superb spatial resolution. Accordingly, it is currently the only imaging modality capable of measuring fibrous cap thickness. Although OCT can ascertain whether lipid is present, it can only provide a semi-quantitative assessment of lipid content. One of the strengths of NIRS is its ability to quantify the amount of lipid present in an automated and rapid fashion.
How does IVUS, as part of the combined catheter, compare to NIRS?
In everyday clinical practice, I find IVUS very helpful for PCI planning, in particular to gauge reference vessel size and lesion length. After stent implantation, IVUS is useful to ensure optimal stent deployment, especially for assessing stent expansion and stent strut apposition. In the STEMI study, we compared the IVUS features of plaque burden and calcification to determine whether IVUS was as good or better than the NIRS in identifying the STEMI culprit site. In a multi-variable analysis, these IVUS characteristics did not improve upon the ability of NIRS to identify the culprit site. In fact, NIRS outperformed IVUS in the analysis. Ultimately, both IVUS and NIRS are complementary to each other. The nice thing about having a combined NIRS-IVUS catheter is that you don’t have to choose one modality or the other — you can use both.
Are you using the NIRS-IVUS catheter in your daily practice?
I am using NIRS-IVUS imaging in almost all of my patients undergoing intervention. This allows me an opportunity to enroll my patients into an ongoing NIRS-IVUS registry at Spectrum Health. I find the NIRS-IVUS images obtained pre-intervention to be helpful for stent selection and to assess the risk of periprocedural infarction. I also use the IVUS imaging after PCI to make sure the stent has adequate expansion and good stent strut apposition before the patient leaves the cath lab.
Disclosure: Dr. Madder reports having received speaker honoraria from Infraredx and has received consulting fees from St Jude Medical.
Dr. Ryan Madder can be contacted at Ryan.Madder@spectrumhealth.org
References and recommended reading
- Madder RD, Goldstein JA, Madden SP, Puri R, Wolski K, Hendricks M, et al. Detection by near-infrared spectroscopy of large lipid core plaques at culprit sites in patients with acute ST-segment elevation myocardial infarction. JACC Cardiovasc Interv. 2013 Aug; 6(8): 838-846. doi: 10.1016/j.jcin.2013.04.012.
- Gardner CM, Tan H, Hull EL, Lisauskas JB, Sum ST, Meese TM, et al. Detection of lipid core coronary plaques in autopsy specimens with a novel catheter-based near-infrared spectroscopy system. JACC Cardiovasc Imaging. 2008 Sep; 1(5): 638-648. doi: 10.1016/j.jcmg.2008.06.001.
- Madder RD, Smith JL, Dixon SR, Goldstein JA. Composition of target lesions by near-infrared spectroscopy in patients with acute coronary syndrome versus stable angina. Circ Cardiovasc Interv. 2012 Feb 1; 5(1): 55-61. doi: 10.1161/CIRCINTERVENTIONS.111.963934.
- Madder RD, Steinberg DH, Anderson RD. Multimodality direct coronary imaging with combined near-infrared spectroscopy and intravascular ultrasound: initial US experience. Catheter Cardiovasc Interv. 2013 Feb; 81(3): 551-557. doi: 10.1002/ccd.23358.
- Dixon SR, Grines CL, Munir A, Madder RD, Safian RD, Hanzel GS, et al. Analysis of target lesion length before coronary artery stenting using angiography and near-infrared spectroscopy versus angiography alone. Am J Cardiol. 2012 Jan 1; 109(1): 60-66. doi: 10.1016/j.amjcard.2011.07.068.










