Evaluation of Coronary Artery Bypass Graft Lesions in the Cath Lab
 Many of our patients undergoing cardiac catheterization have had bypass graft surgery (CABG). Some patients have had surgery over a decade or more ago; others within the last several years. Rarely do patients need to come to the cath lab within six months of their surgery, but it has occurred. I thought it would be worthwhile to discuss what we should think about when assessing the patient with coronary artery bypass graft conduits (saphenous venous grafts [SVG], in-situ internal thoracic [mammary] or free radial artery grafts).
Many of our patients undergoing cardiac catheterization have had bypass graft surgery (CABG). Some patients have had surgery over a decade or more ago; others within the last several years. Rarely do patients need to come to the cath lab within six months of their surgery, but it has occurred. I thought it would be worthwhile to discuss what we should think about when assessing the patient with coronary artery bypass graft conduits (saphenous venous grafts [SVG], in-situ internal thoracic [mammary] or free radial artery grafts).
CABG patients have several reasons to return to the cath lab, but the most common reason is return of a chest pain or anginal syndrome. This syndrome is most often due to 2 mechanisms: 1) progression of CAD in the native arteries or 2) new disease occurring the bypass grafts. The SVG grafts degenerate in 7 to 10 years on average. The SVG lumen accumulates proliferative tissue, as well as atherosclerotic plaques and thrombus over time. This process may or may not limit flow through the graft. It can be expected that the angiography of such a lumen will lead to uncertainty in some patients as to its role in the chest pain syndrome. Internal mammary grafts last more than 20 years, on average, and rarely have intrinsic atherosclerosis. Free radial artery grafts are similar to SVG patency.
Angiographic evaluation of CAB grafts
The first step in the evaluation is to review the operative report, prior catheterization records, and previous angiograms to know how many grafts there are and to what they are attached.
Unfortunately, most of the time, in my experience, getting old records to know where the grafts are is a 50/50 proposition. This makes it important to use an angiographic routine that ensures that one will see all the grafts. The angiographic approach to CABG patients is similar to the assessment of any patient with coronary artery disease, with the addition of aortography, if needed. Either femoral or radial vascular access approach can be used. Left radial access will make engaging a left internal mammary artery (LIMA) graft easier, although much of the time we still use a femoral approach for CABG patients.
Although there is no one right approach, we teach the fellows to image the native coronary arteries first, followed by SVG imaging, IMA imaging, then left ventriculography, and finally, aortography, if we are not satisfied all the grafts have been visualized. Performing aortography after left ventricular (LV) angiography eliminates one catheter exchange. By doing the native coronary arteries first, we get some idea of what territories are supplied by the native vessels and what territories are missing visible arteries that should be supplied by grafts. In addition, observing the contrast washout for competitive flow arising from a functioning graft also provides clues as to location and probable graft patency.
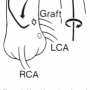 After the native coronary arteriography, we look for the SVG markers (if any) attached to the aorta (Ao) at or near the SVG-Ao anastomosis. It has always been a mystery to me as to why some surgeons use markers and others don’t. They are helpful to the angiographer to some degree, at least, in generally localizing where the ostia are. However, they do not always pinpoint the opening to the graft. SVGs to the left anterior descending artery (LAD) and circumflex (CFX), and their branches are placed on the anterior aortic wall above the left main in the right anterior oblique (RAO) projection and travel toward the apex. Obtuse marginal grafts are usually the highest and farthest left (Figure 1).
After the native coronary arteriography, we look for the SVG markers (if any) attached to the aorta (Ao) at or near the SVG-Ao anastomosis. It has always been a mystery to me as to why some surgeons use markers and others don’t. They are helpful to the angiographer to some degree, at least, in generally localizing where the ostia are. However, they do not always pinpoint the opening to the graft. SVGs to the left anterior descending artery (LAD) and circumflex (CFX), and their branches are placed on the anterior aortic wall above the left main in the right anterior oblique (RAO) projection and travel toward the apex. Obtuse marginal grafts are usually the highest and farthest left (Figure 1).
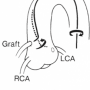 Graft angiography can, in most cases, easily be performed with a right Judkins catheter. In some patients, it may be necessary to use a left coronary vein graft catheter or left Amplatz catheter. For review, the SVG to right coronary artery (RCA) is best engaged in the left anterior oblique (LAO) projection. The SVG is usually located above the RCA ostium and runs parallel with the native RCA. The right coronary vein graft usually can be entered with the right Judkins catheter, often with a simple pullback from the right coronary orifice upward. In a patient with a right vein graft with a down-going takeoff, a right coronary bypass vein graft catheter may be needed. A right (modified) Amplatz catheter also can be used for horizontal or vertical takeoff vein grafts. A circumflex graft is usually located above the LAD vein graft and can be engaged by simply withdrawing the right Judkins catheter upward from the LAD graft orifice (Figure 2).
Graft angiography can, in most cases, easily be performed with a right Judkins catheter. In some patients, it may be necessary to use a left coronary vein graft catheter or left Amplatz catheter. For review, the SVG to right coronary artery (RCA) is best engaged in the left anterior oblique (LAO) projection. The SVG is usually located above the RCA ostium and runs parallel with the native RCA. The right coronary vein graft usually can be entered with the right Judkins catheter, often with a simple pullback from the right coronary orifice upward. In a patient with a right vein graft with a down-going takeoff, a right coronary bypass vein graft catheter may be needed. A right (modified) Amplatz catheter also can be used for horizontal or vertical takeoff vein grafts. A circumflex graft is usually located above the LAD vein graft and can be engaged by simply withdrawing the right Judkins catheter upward from the LAD graft orifice (Figure 2).
Finally, if after searching the anterior and medial aortic walls for a graft orifice or ‘stump’, an aortic root injection should be performed to identify the graft or confirm its absence. A word of caution for SVG angiography: one should avoid unnecessary manipulation of catheters in vein grafts, especially in old grafts that may contain friable atherosclerotic material with a potential risk of embolization.
Internal mammary artery grafts
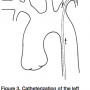 The left internal mammary artery originates anterior from the subclavian artery just beyond the vertebral artery origin (Figure 3). The left subclavian artery can also be entered with a right Judkins catheter, but a mammary artery catheter with a specifically curved tip is preferred. Because there are many variations in the shape of the aortic arch and subclavian artery, it may be difficult to selectively engage the IMA. Non-selective opacification may be needed to visualize the IMA. Avoid vigorous catheter manipulation, because of the risk of artery dissection. The best projection to view the IMA to LAD anastomosis is the true lateral view.
The left internal mammary artery originates anterior from the subclavian artery just beyond the vertebral artery origin (Figure 3). The left subclavian artery can also be entered with a right Judkins catheter, but a mammary artery catheter with a specifically curved tip is preferred. Because there are many variations in the shape of the aortic arch and subclavian artery, it may be difficult to selectively engage the IMA. Non-selective opacification may be needed to visualize the IMA. Avoid vigorous catheter manipulation, because of the risk of artery dissection. The best projection to view the IMA to LAD anastomosis is the true lateral view.
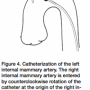
The right internal mammary artery cannulation is less commonly needed and more difficult than that for the left IMA. The right brachiocephalic truncus is entered with use of a right Judkins catheter and is advanced into the subclavian artery. The rest of the manipulation is similar to that described for left internal mammary artery graft cannulation (Figure 4).
 Once angiography of the SVG/IMA grafts is completed and a lesion is identified in the graft or new lesions in the native arteries, a decision for intervention can be made. In the absence of objective evidence of ischemia, especially if there is a question about the SVG lesion(s) or the significance of the native lesion, fractional flow reserve (FFR) can be used. After the decision to proceed, intravascular ultrasound (IVUS) can be used to select best stent size and check apposition after stenting implantation.
Once angiography of the SVG/IMA grafts is completed and a lesion is identified in the graft or new lesions in the native arteries, a decision for intervention can be made. In the absence of objective evidence of ischemia, especially if there is a question about the SVG lesion(s) or the significance of the native lesion, fractional flow reserve (FFR) can be used. After the decision to proceed, intravascular ultrasound (IVUS) can be used to select best stent size and check apposition after stenting implantation.
FFR in SVG lesion assessment
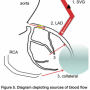 FFR of the SVG is the net result of 3 sources of perfusion to the bed, which is supplied by the SVG. Blood flow to the bed goes through the SVG, the native artery (if not totally occluded), and potentially from the contralateral arteries through collateral flow (Figure 5). Thus, although an SVG lesion appears to be very severe, if the native supply and collateral supply are sufficiently large, the FFR across the SVG could be normal and give reassurance to the team that this risky SVG intervention is unnecessary.
FFR of the SVG is the net result of 3 sources of perfusion to the bed, which is supplied by the SVG. Blood flow to the bed goes through the SVG, the native artery (if not totally occluded), and potentially from the contralateral arteries through collateral flow (Figure 5). Thus, although an SVG lesion appears to be very severe, if the native supply and collateral supply are sufficiently large, the FFR across the SVG could be normal and give reassurance to the team that this risky SVG intervention is unnecessary.
After CABG surgery, the bypass conduit should act in a similar fashion to the native, low resistance epicardial vessel. However, the assessment of ischemia due to a stenosis in a CABG conduit is complicated by several features which include: 1) the potential for competing flow (and pressure) from both the native and conduit vessels; 2) the presence of collaterals from long-standing native coronary occlusion; and 3) the potential for microvascular abnormalities due to ischemic fibrosis and scarring, preexisting or bypass surgery-related myocardial infarction, or chronic low-flow ischemia. Despite these complicating features, the theory of FFR applies just as much to a lesion in a saphenous vein graft to the right coronary artery feeding a normal myocardial bed as a lesion in the native right coronary.
When measuring the FFR across an SVG lesion, the pressure sensor is positioned beyond the anastomosis into the native vessel. If the native vessel is occluded, then the FFR reflects only the SVG lesion. If there is partial flow through the native vessel, then the FFR reflects both the SVG and native vessel lesions. In this case, the SVG and native lesions act like lesions in series and a pressure pullback technique during hyperemia is needed to identify which lesion is producing the most gradient and is responsible for the abnormal FFR. Use IV adenosine for assessing SVG lesions.
FFR and the outcome of SVG after CABG surgery
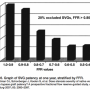 Fractional flow reserve has been applied to study the fate of coronary bypass grafts with striking results. Confirming the relevance of the physiologic stenosis severity and graft patency, Botman et al1 reported the one-year follow up of 164 patients undergoing coronary bypass grafts (n=450). All vessels grafted had FFR measured beforehand with the pressure sensor angioplasty guidewire in the cath lab. At one year, 9% of grafts on functionally significant lesions were occluded, while 21% of grafts on functionally non-significant lesions were occluded (Figure 6). A significant graft occlusion rate was observed for grafted vessels with near normal physiology (FFR > 0.80, normal = 1.0). The angiographic percent diameter narrowings displayed a similar, but less precise, correlation with graft failure. As noted by Botman’s findings, competitive flow between native and grafted coronary vessels, known to surgeons for many years, causes graft failure.2 However, the prediction of graft patency based on the physiology of competitive flow should not be judged by angiography alone. FFR permits objective data on the physiologic impact of intermediately severe stenosis, a finding unknown by angiography, which has tremendous influence on the outcome of the grafts and the patient’s future course.
Fractional flow reserve has been applied to study the fate of coronary bypass grafts with striking results. Confirming the relevance of the physiologic stenosis severity and graft patency, Botman et al1 reported the one-year follow up of 164 patients undergoing coronary bypass grafts (n=450). All vessels grafted had FFR measured beforehand with the pressure sensor angioplasty guidewire in the cath lab. At one year, 9% of grafts on functionally significant lesions were occluded, while 21% of grafts on functionally non-significant lesions were occluded (Figure 6). A significant graft occlusion rate was observed for grafted vessels with near normal physiology (FFR > 0.80, normal = 1.0). The angiographic percent diameter narrowings displayed a similar, but less precise, correlation with graft failure. As noted by Botman’s findings, competitive flow between native and grafted coronary vessels, known to surgeons for many years, causes graft failure.2 However, the prediction of graft patency based on the physiology of competitive flow should not be judged by angiography alone. FFR permits objective data on the physiologic impact of intermediately severe stenosis, a finding unknown by angiography, which has tremendous influence on the outcome of the grafts and the patient’s future course.
I hope this review will be helpful the next time you look at your CABG patient undergoing cath and intervention.
Disclosure: Dr. Kern reports that he is a speaker for Volcano Therapeutics and St. Jude Medical, and is a consultant for Merit Medical.
References
- Botman CJ, Schonberger J, Koolen S, et al. Does stenosis severity of native vessels influence bypass graft patency? A prospective fractional flow reserve-guided study. Ann Thorac Surg 2007;83:2093–2097.
- Sabik JF, Blackstone EH. Coronary artery bypass graft patency and competitive flow. J Am Coll Cardiol 2008:51(2):126-128.










