Endovascular Cooling in STEMI Patients to Reduce Reperfusion Injury: The CHILL-MI Study
[Editor's note: CHILL-MI enrollment is now complete (April 30, 2013)]
 CHILL-MI is a prospective, randomized, multi-center study designed to further evaluate the safety and effectiveness of Philip’s InnerCool RTx endovascular cooling system in patients suffering from ST-elevation myocardial infarction (STEMI). The study will enroll 120 patients at multiple sites in Europe with a primary endpoint of reducing infarct size as a percentage of myocardium at risk, as assessed by cardiac magnetic resonance imaging (MRI). The study will also reinforce the safety and efficacy data demonstrated in the RAPID MI-ICE trial in a larger patient group. The RAPID MI-ICE trial showed a 38% reduction in infarct size in STEMI patients who were cooled to a temperature of <35° Celsius (C) prior to performing angioplasty.
CHILL-MI is a prospective, randomized, multi-center study designed to further evaluate the safety and effectiveness of Philip’s InnerCool RTx endovascular cooling system in patients suffering from ST-elevation myocardial infarction (STEMI). The study will enroll 120 patients at multiple sites in Europe with a primary endpoint of reducing infarct size as a percentage of myocardium at risk, as assessed by cardiac magnetic resonance imaging (MRI). The study will also reinforce the safety and efficacy data demonstrated in the RAPID MI-ICE trial in a larger patient group. The RAPID MI-ICE trial showed a 38% reduction in infarct size in STEMI patients who were cooled to a temperature of <35° Celsius (C) prior to performing angioplasty.
Research has shown that the key to reducing infarct size is to cool patients with therapeutic hypothermia to <35°C prior to reperfusion. Patients in the CHILL-MI treatment group will receive endovascular cooling therapy in combination with 1-2 L of cold saline. The control group will receive the current standard of care. The primary endpoint will be the myocardial infarct size as a percentage of at-risk myocardium at 4 days (± 2 days), as measured by cardiac MRI. Secondary safety and efficacy endpoints will also be evaluated.
Prior to CHILL-MI, the smaller RAPID MI-ICE study enrolled 20 STEMI patients who were randomized to receive immediate percutaneous coronary intervention with or without adjunct rapid endovascular cooling. The study demonstrated that the induction of mild hypothermia (<35°C) in STEMI patients prior to performing an angioplasty can save up to 38% more heart tissue than the current standard of care. Data showed that, in the control group, more patients experienced heart failure (n=3) and ventricular tachycardia/ventricular fibrillation (VT/VF; n=2) than in the endovascular cooling group, which had no instance of either complication (p=0.21). Three patients in the endovascular cooling group experienced infections, while no patients in the control group had infections (p=0.21). The conclusion that the rapid induction of hypothermia with endovascular catheter is safe and feasible in patients with acute MI prompted the initiation of the CHILL-MI study to investigate this technology in a larger multi-center trial.
Dr. Götberg, why use hypothermia on STEMI patients?
We know that opening the vessel is paramount for patients with acute myocardial infarction and of course we want to do it as fast as possible. But what we are trying to figure out in CHILL-MI is how to protect the heart further. When the closed vessel is opened, additional damage is incurred to the heart. This phenomenon is called reperfusion injury. Every interventionist knows that the patient experiences additional chest pain and a drop in blood pressure upon reperfusion. Hypothermia is a means to try and reduce the extent of myocardial reperfusion injury; in other words, reduce the size of the myocardial infarction. Experimental results have shown a reduction in infarct size by ≈ 40%. In my opinion, it is the most promising method of cardioprotection currently under investigation.
How does hypothermia for STEMI patients compare to hypothermia used in patients with cardiac arrest?
People are often confused by the difference. The goal of cooling an unconscious patient who has survived a cardiac arrest is to salvage the brain. In CHILL-MI, we have awake patients with a STEMI. We are cooling the patient in order to salvage myocardium.
How does endovascular cooling for STEMI patients work?
If you cool someone, regardless of whether they are unconscious or conscious, they are prone to shiver, because it is natural for the body to try and keep its temperature at 37˚C. A previous small trial was performed on awake STEMI patients where researchers tried to apply external cooling, but they started to shiver very quickly, which limited the efficacy of the treatment. The whole “trick” with the endovascular cooling method is cooling from within. It turns out that endovascular cooling does not activate the skin sensory receptors to the same extent as external cooling. Oddly enough, cooling from within doesn’t make the patient feel as cold as the use of external cooling. If you think about it, this is actually quite logical, because the body regulates temperature in response to the outside environment. So the body doesn’t “realize” when we cool from within; endovascular cooling doesn’t activate as many receptors, which reduces the amount of shivering. Unconscious cardiac arrest patients receive certain drugs to keep their body from shivering and counteracting the hypothermia treatment. We also administer two medications that have been shown in previous trials to reduce shivering. Buspirone is a drug normally used to reduce anxiety; in this setting, it is given as two pills. The other is meperidine (Demerol), which is a morphine-like substance, administered by intermittent injection. Both these medications eliminate the tendency to shiver.
How does the InnerCool RTx cooling system work?
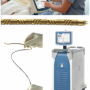 The system consists of a 14 French (F) catheter made of a flexible metal. Inside the catheter, in a closed circuit, cold saline is circulated, effectively cooling the entire catheter. It is basically a heat-exchange catheter. It takes about three to four minutes to insert, done through a femoral vein, utilizing the same technique for putting in a temporary pacemaker, which every cath lab physician knows how to do. It is a very simple method of insertion and once it is in place, you just push a button on the console, and the cold saline starts to circulate inside the catheter. Because the catheter is placed through the femoral vein to the inferior vena cava, a large volume of blood circulates around the catheter. Most of the blood in the circulation goes past this catheter and is cooled. It is very efficient. Since the catheter is put in a vein, when the sheath is removed, only mild compression to the groin is required.
The system consists of a 14 French (F) catheter made of a flexible metal. Inside the catheter, in a closed circuit, cold saline is circulated, effectively cooling the entire catheter. It is basically a heat-exchange catheter. It takes about three to four minutes to insert, done through a femoral vein, utilizing the same technique for putting in a temporary pacemaker, which every cath lab physician knows how to do. It is a very simple method of insertion and once it is in place, you just push a button on the console, and the cold saline starts to circulate inside the catheter. Because the catheter is placed through the femoral vein to the inferior vena cava, a large volume of blood circulates around the catheter. Most of the blood in the circulation goes past this catheter and is cooled. It is very efficient. Since the catheter is put in a vein, when the sheath is removed, only mild compression to the groin is required.
What is the temperature level you are aiming for?
We are aiming for 33˚C. That is our target temperature. But we also realize that it is very difficult to reach 33˚C before the time of reperfusion. Animal studies and subgroup analysis of two clinical trials have shown that as long as the patient is below 35˚C at the time of reperfusion, the hypothermia treatment has a beneficial effect. When patients come in, they usually have a temperature of around 37˚C. We give them intravenous cold saline, insert the endovascular cooling catheter, and perform the angiogram. When we cross the lesion, the temperature is measured, and all patients have been below 35˚C thus far.
RAPID-MI-ICE was the first, smaller study of hypothermia in STEMI patients using a combination of two hypothermia methods. What did you learn?
No one wants to delay reperfusion, and in previous studies using endovascular cooling alone, more than half of the patients had not reached a temp below 35˚C at the time of reperfusion. We performed additional animal studies and saw that a combination of cold saline and endovascular cooling was very efficient. We then performed the RAPID-MI ICE study and evaluated endovascular cooling together with a cold saline bolus, using about 1,000mL of cold saline infused through a peripheral vein. These two methods combined allowed the patient to obtain a temperature below 35˚C, we then continued to cool with the goal of reaching 33˚C, which generally took an additional 30 minutes. So from the initial patient temperature of 37˚C, it takes about 15-30 minutes to get below 35˚C, and then an additional 30 minutes to get below 33˚C. Timepoint zero was the administration of cold saline while the patient was prepared and dressed. About 10-15 minutes later, we inserted the endovascular cooling catheter, performed the angiogram and then continued with the PCI. The hypothermia treatment caused about a 3-minute delay in reperfusion compared to control.
What were the benefits seen in RAPID-MI ICE that led to CHILL-MI?
In the small pilot trial, we saw a 38% reduction in infarct size compared to the control group, with no major safety issues.
The cooling system remains in place during reperfusion?
Yes. In the previous trial, the RAPID-MI ICE trial, it was in place for three hours past reperfusion, because we didn’t truly know how long we needed to cool the patient. We then performed further animal studies that suggested that one hour cooling past reperfusion should be enough. In the CHILL-MI trial, we are thus cooling for one hour past reperfusion. That is one simplification of the cooling protocol in the CHILL-MI trial. A patient comes in with a STEMI, cooling is started in the cath lab, and PCI is performed. Cooling is then discontinued and and the catheter is removed at the cath lab. The patient is then transported in a bed to the cardiac care unit.
So the patient stays in the cath lab for the additional cooling time and you remove the catheter?
Since this procedure is part of a study, we want to keep it as simple as possible and perform the cooling in the cath lab. It would still be easy to transfer the cooling console and the patient to the ward, plug it in and continue cooling for the remainder of time. In the RAPID-MI-ICE trial, because the cooling time was 3 hours, we did transfer patients to the ward.
Aside from the shivering response, how are patients tolerating endovascular cooling?
When using buspiron and meperidine to prevent shivering, the patients tolerate the treatment very well. The patients feel fine during the circumstances, meaning they feel and behave pretty much like any STEMI patient. Some may experience a mild discomfort, saying, “I feel a little bit cold.” We then usually give them an additional blanket. If we warm patients up a little externally, they feel fine. The cold receptors in the skin are not activated to the same extent when cooling internally. It is important, though, to keep them warm on the outside to prevent cold receptor activation and shivering.
How is the emergency department involved (or not) with cooling?
 For about 10 years now, our region has had a wireless ECG system in all ambulances. In all patients with chest pain, an ECG is transferred to a cardiologist at the cardiac care unit for interpretation. If the ECG shows a STEMI, the ambulance is directed to go directly to the cath lab. We have about 700 STEMI patients/year at our cath lab. Each one, with very few exceptions, is directly transferred to the cath lab, effectively bypassing the ER. We consider it a failure if we do find a patient at the ER, because it means a significant delay in door-to-balloon time.
For about 10 years now, our region has had a wireless ECG system in all ambulances. In all patients with chest pain, an ECG is transferred to a cardiologist at the cardiac care unit for interpretation. If the ECG shows a STEMI, the ambulance is directed to go directly to the cath lab. We have about 700 STEMI patients/year at our cath lab. Each one, with very few exceptions, is directly transferred to the cath lab, effectively bypassing the ER. We consider it a failure if we do find a patient at the ER, because it means a significant delay in door-to-balloon time.
Is staff on call or do you have everyone on site already?
The physicians are usually on call, and since we know of a STEMI when the patient is in the ambulance, we usually have a 20-30 minutes transfer time before the patient arrives to the cath lab. The hospital just calls the physician, and he drives to the hospital. We have on-site staff at the cath lab or the coronary care unit.
Is there any additional staff required with the endovascular cooling procedure?
Our staff for a STEMI patient includes an interventional cardiologist, two nurses, and a cath lab tech. No additional staff is required for this study.
What was the learning curve like?
It was a very short learning curve to learn how to apply hypothermia in awake patients. All interventionists already know how to insert a femoral catheter. We quickly learned that it was more efficient to give the patient the anti-shivering drugs before he/she started shivering. In other words, to act rather than react to prevent shivering from reducing the efficacy of the hypothermia treatment. If the CHILL-MI trial is positive, it would be very easy to implement endovascular cooling into pretty much any cath lab setting with only minor training.
Where do things stand now with the CHILL-MI study?
We are still early in the study. We have enrolled nine patients out of 120, and have 10 centers participating all around Europe. Skane University Hospital is the first participating center — all the other centers will start enrolling patients within a few weeks, so enrollment will speed up soon. We expect to have a quick enrollment phase, and have projected that we will conclude the trial in six months.
There are some other methods are being investigated to reduce reperfusion injury in STEMI and many of these methods would delay the opening of the vessel and/or would require extensive training. One of the advantages of hypothermia, should the CHILL-MI trial be positive, is its simplicity. It is very easy to put in the catheter, and I wouldn’t expect any complications from it. In the RAPID-MI ICE trial, it took us only three additional minutes to set up the console and insert the catheter when compared to the control group that wasn’t subjected to hypothermia.
Dr. Götberg can be contacted at matthias.gotberg@med.lu.se.
Disclosure: Dr. Götberg reports no conflicts of interest regarding the content herein.
For Further Reading
- Götberg M, van der Pals J, Götberg M, et al. Optimal timing of hypothermia in relation to myocardial reperfusion. Basic Res Cardiol 2011 Sep;106(5):697-708.
- Götberg M, Olivecrona GK, Koul S, et al. A pilot study of rapid cooling by cold saline and endovascular cooling before reperfusion in patients with ST-elevation myocardial infarction. Circ Cardiovasc Interv 2010 Oct;3(5):400-407.
- Götberg M, Olivecrona GK, Engblom H, et al. Rapid short-duration hypothermia with cold saline and endovascular cooling before reperfusion reduces microvascular obstruction and myocardial infarct size. BMC Cardiovasc Disord 2008 Apr 10;8:7.










