Coronary Myocardial Bridging in Noonan Syndrome
Abstract
We describe a 37-year-old male with a history of Noonan syndrome, confirmed via genetic testing in childhood, presenting with chest pain. A 12-lead ECG showed ST-segment depression in the lateral chest leads, and serial troponin values were elevated. The patient was diagnosed with NSTEMI. Two-dimensional echocardiography showed hypertrophic cardiomyopathy. Coronary angiography revealed significant myocardial bridging of the middle segment of the left anterior descending coronary artery, with no other abnormalities. This finding is the cause of the patient’s symptoms.
testing in childhood, presenting with chest pain. A 12-lead ECG showed ST-segment depression in the lateral chest leads, and serial troponin values were elevated. The patient was diagnosed with NSTEMI. Two-dimensional echocardiography showed hypertrophic cardiomyopathy. Coronary angiography revealed significant myocardial bridging of the middle segment of the left anterior descending coronary artery, with no other abnormalities. This finding is the cause of the patient’s symptoms.
Introduction
Noonan syndrome is an autosomal dominant disorder characterized by minor facial dysmorphism, webbed neck, proportionate short stature, cryptorchidism and congenital heart diseases.1 The most commonly seen defects are dysplastic pulmonary valve and hypertrophic cardiomyopathy.2 There are only two cases of Noonan syndrome concomitant with myocardial bridging reported in the literature. Herein, we report a case of chest pain related to myocardial bridging in a patient with Noonan syndrome featuring hypertrophic cardiomyopathy.
Case report
A 37-year-old Hispanic male with a past medical history of grand mal seizures and Noonan syndrome presented to the emergency department complaining of substernal chest pain. He described the chest pain as non-radiating tightness that started while he was moving furniture at home, lasted for 15 minutes, and spontaneously resolved. He denied shortness of breath, dizziness, fainting or palpitations.
On physical exam, 3/6 harsh crescendo-decrescendo systolic murmur was heard on the left lower sternal border. Physical exam of the head and neck showed hypertelorism, low-set posteriorly rotated ears, downward eye slants, and webbed neck. A 12-lead ECG showed sinus rhythm at 98 beats per minute and left axis deviation with ST-segment depression in lateral chest leads, concerning for possible lateral ischemia or infarct. Three sets of troponin (I) were checked sequentially with 6-hour intervals and the results were (0.03), (12.50), (11.33); normally (<0.01). The patient was managed as a non-ST-segment elevation myocardial infarction (NSTEMI). Subsequent 2D echocardiogram showed moderate asymmetric septal and left ventricular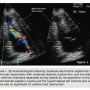 hypertrophy with moderate diastolic dysfunction, and two distinct left ventricle chambers separated during systole by the apposition of the hypertrophied papillary muscle and the hypertrophied left ventricle (LV) wall (Figure 1). There was no significant LV outflow tract obstruction.
hypertrophy with moderate diastolic dysfunction, and two distinct left ventricle chambers separated during systole by the apposition of the hypertrophied papillary muscle and the hypertrophied left ventricle (LV) wall (Figure 1). There was no significant LV outflow tract obstruction.
Cardiac catheterization was performed. Coronary angiography showed significant myocardial bridging across the middle segment of the left anterior descending artery (LAD) (Figures 2A-B). Contrast ventriculography confirmed the systolic mid-LV obstruction. Surgery was offered; however, the patient declined surgery and was treated conservatively with a beta blocker and calcium channel blocker.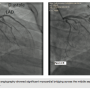
Discussion
Noonan syndrome is an autosomal dominant disorder with an estimated incidence of one in 1000 to 2500 live births. Approximately 50% of children with Noonan syndrome have a mutation in the PTPN11 gene, mapped to chromosome 12q24.1, which encodes the non-receptor protein tyrosine phosphatase SHP2.3 Less common mutations have been described in KRAS, SOS1, and NRAS, which like PTPN11 result in up-regulation of the RAS-MAP kinase pathway.4,5 Noonan syndrome is characterized by minor facial dysmorphism (hypertelorism, anti-mongoloid slant of the palpebral fissures with ptosis, low-set ears and thickened helix), webbed neck, proportionate short stature, cryptorchidism, and congenital heart disease.1
Cardiac defects associated with Noonan syndrome were assessed by Brush et al2 in a study that involved 118 patients confirmed to have a Noonan genetic phenotype. The pulmonary valve appeared frankly dysplastic in eight patients (7%) and was associated with significant stenosis in six (75%) of the eight. In the 110 patients without obvious dysplasia, significant stenosis was present in 22 (20%). Left ventricular hypertrophy was present in 29 patients (25%) without significant pulmonary stenosis. Localized anterior septal hypertrophy was the most common pattern in 12 (41%) of 29 patients. Diffuse hypertrophy involving the entire septum and the free wall was present in nine patients (31%) and was severe (>1.7 cm) in five. Myocardial bridging has only been mentioned in association with Noonan syndrome in two case reports.6,7
Myocardial bridging is a congenital anomaly in which major coronary arteries, normally distributed over the epicardial surface of the heart, have segmental intramyocardial courses.8 During systole, this segment of the vessel is compressed, a condition referred to as milking or systolic “myocardial bridging.”9 Myocardial bridging was first recognized more than 200 years ago,10 reported in depth in 1951, and recognized angiographically in 1960. In 90% of cases, bridging is localized to the middle segment of the LAD.10,11 It is generally a benign condition. There are few reports of survival rates, but when studied, five-year survival ranges between 85 and 98%.12 Myocardial bridging usually causes coronary artery obstruction only during systole, something not expected to significantly reduce total myocardial perfusion, since almost two-thirds of blood flow in the left coronary system occurs in diastole, most of which is directed to the sub-endocardial layer. However, sometimes persistent coronary narrowing in early diastole occurs, the so-called “spill over” phenomenon, which may lead to myocardial ischemia and, on rare occasions, acute myocardial infarction or sudden death. In tachycardia, during which systole will occupy a greater percentage of the cardiac cycle, a shortening of the diastolic filling period will contribute to greater systolic compression. The presence of myocardial bridging can sometimes initiate development of an atherosclerotic lesion or facilitate progression of atherosclerosis in the proximal segment of the vessel.13,14
Our patient was in sinus rhythm when he presented to the emergency department, with a heart rate of 98 beats per minute. However, he was moving furniture when his pain started, which made stress-induced tachycardia a more likely culprit for exacerbating his bridging-induced ischemia and, thus, causing his symptoms, as no atherosclerotic plaque was seen on angiography.
The reported prevalence of bridging varies according to the method of evaluation. Pathologic studies have found a mean frequency of myocardial bridging of 25% (range 5 to 86%), similar to that observed in noninvasive imaging studies using coronary computed tomography (CT).15 On the other hand, using coronary angiography, the reported prevalence of myocardial bridging is 1.7% (range 0.5 to 16%), and is almost always confined to the LAD.16 Some studies showed that the depiction rate of myocardial bridging is greater with CT coronary angiography than with conventional coronary angiography.17 A higher prevalence of myocardial bridging has been observed in patients with hypertrophic cardiomyopathy (HCM) and in recipients of cardiac transplants.8,12
Annual mortality in HCM is 2 to 6%, primarily from sudden cardiac death, due to arrhythmias or myocardial ischemia, and occasionally related to myocardial bridging. In an angiographic study of 36 children with HCM done by Yetman et al18, 10 (28%) had myocardial bridging of the LAD persisting for 50% of diastole. Compared to patients without bridging, those with bridging had a significantly greater incidence of chest pain (60 versus 19%), history of resuscitated cardiac arrest (50 versus 4%), and ventricular tachycardia on ambulatory monitoring (80 versus 8%).
A later report evaluated 57 children with HCM, 23 of which (40%) had myocardial bridging. Bridging was associated with more severe left ventricular hypertrophy, but not ischemia or sudden death.19
Symptomatic patients with myocardial bridging can be treated medically with beta blockers and possibly non-dihydropyridine calcium channel blockers, because these agents reduce heart rate and myocardial contractility, and therefore, normalize the systolic/diastolic flow ratio. Nitrates, on the other hand, reduce the intrinsic coronary wall tension and increase the reflex sympathetic stimulation of contractility, and may worsen symptoms. Therefore, their use is felt to be contraindicated.9
Preliminary studies suggest that intracoronary stent placement may normalize disturbed intracoronary hemodynamics and improve clinical symptoms in patients with symptomatic bridging.20,21 However, myocardial bridging may negatively affect endothelial function. The turbulent shear stress and intimal trauma predispose the vessel toward thrombus formation. Therefore, myocardial bridging might be a potential risk factor of very late stent thrombosis of drug-eluting stents.22,23 Another associated complication is stent fracture leading to stent thrombosis.24,25 Coronary rupture during stent implantation has also been reported in patients who had myocardial infarction caused by coronary bridging.26-28 It has been postulated that rupture might occur because of the thin intima of the bridged artery, thin myocardial layer toward the right ventricle, and smaller LAD diameter in the bridged segment.26
Surgical therapy should be reserved for patients with persistent symptoms and in whom ischemic changes are proven, as well as those with a high-risk marker (such as life-threatening ventricular arrhythmias, aborted sudden death, or nonfatal myocardial infarction) in whom a trial of medical therapy has failed. The operative procedure of choice is resection of the muscle bridge using cardiopulmonary bypass.29-31 Dissection of the overlying myocardial fibers with complete exposure of the coronary artery is essential if recurrence is to be avoided.
Conclusion
Myocardial bridging, although generally an incidental finding, should be considered as a cause of chest pain or myocardial ischemia in patients with a history of Noonan syndrome, especially those with associated HCM. Diagnosis may be established with percutaneous coronary angiography or CT coronary angiography. The preferred treatment is medical therapy with beta blockers and/or non-dihydropyridine calcium channel blockers. Surgical resection of the muscle bridge should be considered in patients with life-threatening ventricular arrhythmias, aborted sudden death, and failure of medical therapy.
This article received a double-blind peer review from members of the Cath Lab Digest editorial board.
The authors can be contacted via Dr. Hazem Alhawasli at drhawasly@hotmail.com.
References
- Allanson JE. Noonan syndrome. J Med Genet. 1987; 24(1): 9-13.
- Burch M, Sharland M, Shinebourne E, Smith G, Patton M, McKenna W. Cardiologic abnormalities in Noonan syndrome: phenotypic diagnosis and echocardiographic assessment of 118 patients. J Am Coll Cardiol. 1993; 22(4): 1189-1192.
- Tartaglia M, Mehler EL, Goldberg R, Zampino G, Brunner HG, Kremer H, et al. Mutations in PTPN11, encoding the protein tyrosine phosphatase SHP-2, cause Noonan syndrome. Nat Genet. 2001; 29(4): 465-468.
- Roberts AE, Araki T, Swanson KD, Montgomery KT, Schiripo TA, Joshi VA, et al. Germline gain-of-function mutations in SOS1 cause Noonan syndrome. Nat Genet. 2007; 39(1): 70-74.
- Schubbert S, Zenker M, Rowe SL, Böll S, Klein C, Bollag G, et al. Germline KRAS mutations cause Noonan syndrome. Nat Genet. 2006; 38(3): 331-336.
- Martínez-Quintana E, Rodríguez-González F, Junquera-Rionda P. Noonan syndrome and different morphologic expressions of hypertrophic cardiomyopathy. Pediatr Cardiol. 2012 Jul 13. [Epub ahead of print]
- Leye M, Calcagni G, Brunelle F, Bonnet D, Sidi D, Ou P. Coronary myocardial bridging in Noonan syndrome: definitive diagnosis with high-resolution CT. Br J Radiol. 2009 Jan; 82(973): e8-e10.
- Möhlenkamp S, Hort W, Ge J, Erbel R. Update on myocardial bridging. Circulation. 2002;106(20):2616-2622.
- Alegria JR, Herrmann J, Holmes DR Jr, Lerman A, Rihal CS. Myocardial bridging. Eur Heart J. 2005; 26(12): 1159-1168.
- Reyman, HC. Disertatis de vasis cordis propis. Bobl Anatomy. 1737; 2: 368.
- Laurent G, Cottin Y, André F, Pichon E, Piszker G, Gérard C, Gabrielle F, Ravisy J, Louis P, Wolf JE. [Symptomatic myocardial bridges. Apropos of 6 cases]. Arch Mal Coeur Vaiss. 1996 Jul; 89(7): 883-887.
- Sorajja P, Ommen SR, Nishimura RA, Gersh BJ, Tajik AJ, Holmes DR. Myocardial bridging in adult patients with hypertrophic cardiomyopathy. J Am Coll Cardiol. 2003; 42(5): 889-894.
- Schunkert H. Focal coronary atherosclerosis proximal to myocardial bridging. Circulation. 2003 Apr 15; 107(14): 1944.
- Sun JL, Huang WM, Guo JH, Li XY, Ma XL, Wang CY. Relationship between myocardial bridging and coronary arteriosclerosis. Cell Biochem Biophys. 2013 Apr; 65(3): 485-489.
- Konen E, Goitein O, Sternik L, Eshet Y, Shemesh J, Di Segni E. The prevalence and anatomical patterns of intramuscular coronary arteries: a coronary computed tomography angiographic study. J Am Coll Cardiol. 2007 Feb 6; 49(5): 587-593.
- Ge J, Erbel R, Rupprecht HJ, Koch L, Kearney P, Görge G, Haude M, Meyer J. Comparison of intravascular ultrasound and angiography in the assessment of myocardial bridging. Circulation.1994; 89(4): 1725-1732.
- Leschka S, Koepfli P, Husmann L, Plass A, et al. Myocardial bridging: depiction rate and morphology at CT coronary angiography — comparison with conventional coronary angiography. Radiology. 2008 Mar; 246(3): 754-762.
- Yetman AT, McCrindle BW, MacDonald C, Freedom RM, Gow R. Myocardial bridging in children with hypertrophic cardiomyopathy – a risk factor for sudden death. N Engl J Med. 1998; 339(17): 1201-1209.
- Mohiddin SA, Begley D, Shih J, Fananapazir L. Myocardial bridging does not predict sudden death in children with hypertrophic cardiomyopathy but is associated with more severe cardiac disease. J Am Coll Cardiol. 2000; 36(7): 2270-2278.
- Klues HG, Schwarz ER, vom Dahl J, Reffelmann T, Reul H, Potthast K, et al. Disturbed intracoronary hemodynamics in myocardial bridging: early normalization by intracoronary stent placement. Circulation. 1997; 96(9): 2905-2913.
- Prendergast BD, Kerr F, Starkey IR. Normalization of abnormal coronary fractional flow reserve associated with myocardial bridging using an intracoronary stent. Heart. 2000; 83(6): 705-707.
- Tsujita K, Maehara A, Mintz GS, Doi H, Kubo T, Castellanos C. Impact of myocardial bridge on clinical outcome after coronary stent placement. Am J Cardiol. 2009; 103(10): 1344-1348.
- Rondan J, Lozano I, Avanzas P, Lopez-Palop R, Vegas JM, Moris C. Drug-eluting stents may not be the answer for myocardial bridges. Int J Cardiol. 2007 Apr; 117(2): e76-e78.
- Tandar A, Whisenant BK, Michaels AD. Stent fracture following stenting of a myocardial bridge: report of two cases. Catheter Cardiovasc Interv. 2008 Feb; 71(2): 191-196.
- Srinivasan M, Prasad A. Metal fatigue in myocardial bridges: stent fracture limits the efficacy of drug-eluting stents. J Invasive Cardiol. 2011 Jun; 23(6): E150-E152.
- Tomasevic M, Dikic M, Ostojic M. Stenting a myocardial bridge: a wrong decision in STEMI? Acta Cardiol. 2011 Feb; 66(1): 89-91.
- Hering D, Horstkotte D, Schwimmbeck P, Piper C, Bilger J, Schultheiss HP. Acute myocardial infarct caused by a muscle bridge of the anterior interventricular ramus: complicated course with vascular perforation after stent implantation. Z Kardiol. 1997 Aug; 86(8): 630-638.
- Shen TY, Chen CC, Tseng YZ. Stent graft used to rescue coronary rupture during percutaneous coronary intervention for myocardial bridge. Intern Med. 2009; 48(12): 993-996.
- Haager PK, Schwarz ER, vom Dahl J, Klues HG, Reffelmann T, Hanrath P. Long term angiographic and clinical follow up in patients with stent implantation for symptomatic myocardial bridging. Heart. 2000; 84(4): 403-408.
- Hill RC, Chitwood WR Jr, Bashore TM, Sink JD, Cox JL, Wechsler AS. Coronary flow and regional function before and after supraarterial myotomy for myocardial bridging. Ann Thorac Surg. 1981; 31(2): 176-181.
- Iversen S, Hake U, Mayer E, Erbel R, Diefenbach C, Oelert H. Surgical treatment of myocardial bridging causing coronary artery obstruction. Scand J Thorac Cardiovasc Surg. 1992; 26(2): 107-111.
______________________________
*Internal Medicine Resident, Saint Joseph Hospital – UIC affiliated, Chicago, Illinois; †Internal Medicine Residency Program Director, Clinical Associate Professor of Medicine, University of Illinois College of Medicine, Chicago, Saint Joseph Hospital, Chicago, Illinois; ‡Assistant Professor of Clinical Medicine, Northwestern University Feinberg School of Medicine, Saint Joseph Hospital, Chicago, Illinois










