ADVERTISEMENT
The Cardiovascular Hybrid Surgical Room: Evolving into the Future of Cardiovascular Surgery
This article is adapted by the author from Kpodonu J. The Cardiovascular Hybrid Room. In: Kpodonu J. Manual of Thoracic Endoaortic Surgery. London, England: Springer; 2010.
The last few years have seen a paradigm shift in the treatment of cardiovascular-related diseases, from once traditional open surgical modalities to the entire cardiovascular tree being amenable to percutaneous interventions. The tremendous advances in transcatheter endovascular procedures currently being applied to the heart and the peripheral vasculature have resulted in a treatment paradigm shift in the care of the cardiovascular patient. To allow these procedures, operating rooms with integrated x-ray imaging capabilities have to be installed. Because of their size and complexity, these integrated endovascular suites, or hybrid operating rooms, require special considerations, planning and design, as well as new skills to be learned by the team. Guidelines on hybrid ORs are currently missing but are under development by multidisciplinary teams.
The new operative environment (the hybrid surgical suite)
A fully integrated interventional suite combines surgical sterility1,2 with flat-panel cardiovascular imaging, a linked workstation, post processing and storage facilities.3 The size of the hybrid room should be of sufficient dimensions to allow anesthesiology facilities needed for full patient monitoring. Furthermore, any type of supportive equipment available in the room, such as machinery required for intravascular ultrasound, three-dimensional (3D) transesophageal echocardiogram or rotational angiography, the ability for open conversion or hybrid intervention, as well as endovascular supplies and devices, must all be able to fit in the hybrid suite. Current peripheral suites are fitted with many interesting features to make certain procedures easier. An on-table duplex ultrasound makes puncturing of the vessel easy and is a good guide during percutaneous access. The possibility of storing several reference points to which the C-arm can be automatically relocated at any time during the procedure facilitates the management of even extremely complex procedures. It is obvious that routine endovascular and open surgical practice both clearly gain from performance in this dual-capability working environment. For example, classic open bypass creation is immediately controlled on-table. When improvement of inflow or outflow becomes necessary after bypass surgery, balloon dilation with or without additional stent placement can be rapidly performed without dramatically prolonging procedural time. The use of an integrated endovascular suite, however, stretches beyond hybrid procedures and opens doors to new diagnostic and treatment possibilities. Three-dimensional reconstructions generated by integrated computed tomography (CT) or rotational angiography can make a real-time visualization of vessel morphology in any direction and improve the visibility of vessel structures. Application of 3D reconstruction during treatment of intracranial aneurysms, for instance, is a must to ensure optimal positioning of catheters, coils, balloons and stents. An integrated setting means saving time and personnel, because more procedures can be completed in the same room by the existing staff without increasing the strain on the team and without relocating equipment or personnel from another department. Unique technology in the suite allows physicians across different specialties to work together on a case-by-case scenario, in the best interest of each patient. The most advanced imaging systems available provide quick and detailed information for shorter, more accurate treatment with substantially less x-ray exposure when compared to traditional devices. Complex cases are more easily treated, since the suite is designed to handle both minimally invasive, percutaneous hybrid operations and open surgical procedures.
Basic equipment and design of the hybrid endovascular operating room
The primary components of the hybrid suite center are intra-operative angiography and fluoroscopy, as well as carefully designed operating tables to accommodate and optimize the usefulness of the radiographic equipment. The hybrid suite’s imaging system provides superior image quality, higher tube heat capacity, and has measurement capabilities capable of simple and complex procedures requiring high resolution. The price range is between $2-7 million depending on the brand, specifications, and ability to provide rotational angiography. The cost could be even higher with the addition of a biplanar system and with integration of various sophisticated imaging modalities, like 3D echocardiography, intra-cardiac ultrasound, intravascular ultrasound or electromagnetic navigation systems.
Size of the hybrid operating room and radiation
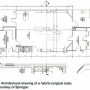 The operating room should ideally be between 1,000-1,200 square feet with a minimum clear area of 400-500 square feet (Figure 1). Floor to ceiling heights should be at a minimum of 10 feet to accommodate floor or ceiling-mounted C-arms capable of rotational angiography for volume rendering 3D CT-like images achieved with advanced robotic imaging systems (Figure 2). In existing operating rooms, the fixed, ceiling-mounted C-arm requires some structural modifications to install the mounting plates and run electrical conduits under the floor to the components. Most states dictate that any operating room with a fixed imaging system must have lead-lined walls. Most standard ORs have a leaded covering of 0.5 mm that is not sufficient for the radiation dose generated by a fixed unit. Lead-lined walls in the range of 2-3 mm for the fixed units may be needed and may vary from state to state (for example, see regulations for the state of California). The expense of these constructions /modifications can vary up to $100,000, depending on the original condition of the room, local contracting costs and architect’s fees.
The operating room should ideally be between 1,000-1,200 square feet with a minimum clear area of 400-500 square feet (Figure 1). Floor to ceiling heights should be at a minimum of 10 feet to accommodate floor or ceiling-mounted C-arms capable of rotational angiography for volume rendering 3D CT-like images achieved with advanced robotic imaging systems (Figure 2). In existing operating rooms, the fixed, ceiling-mounted C-arm requires some structural modifications to install the mounting plates and run electrical conduits under the floor to the components. Most states dictate that any operating room with a fixed imaging system must have lead-lined walls. Most standard ORs have a leaded covering of 0.5 mm that is not sufficient for the radiation dose generated by a fixed unit. Lead-lined walls in the range of 2-3 mm for the fixed units may be needed and may vary from state to state (for example, see regulations for the state of California). The expense of these constructions /modifications can vary up to $100,000, depending on the original condition of the room, local contracting costs and architect’s fees.
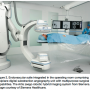 As endovascular/cardiovascular hybrid surgical procedures become more complex, the relationship of the C-arm, table and patient’s position becomes even more important. The fluoroscopic unit should be able to move in a horizontal plane from the groin along the course of the vessel with the ability to “snap images on the move.” This parallel movement prevents the need for excessive contrast material and greatly expedites the procedure. When a catheter is placed in the brachial artery, the fluoroscopic unit must be capable of rapid movement over the catheter’s path from the arm and though the thoracic aorta to the area of ultimate instrumentation. Obstructions from a table or a floor-mounted portable unit that hinders rapid panning over wide anatomic areas limit potential success of the procedure.
As endovascular/cardiovascular hybrid surgical procedures become more complex, the relationship of the C-arm, table and patient’s position becomes even more important. The fluoroscopic unit should be able to move in a horizontal plane from the groin along the course of the vessel with the ability to “snap images on the move.” This parallel movement prevents the need for excessive contrast material and greatly expedites the procedure. When a catheter is placed in the brachial artery, the fluoroscopic unit must be capable of rapid movement over the catheter’s path from the arm and though the thoracic aorta to the area of ultimate instrumentation. Obstructions from a table or a floor-mounted portable unit that hinders rapid panning over wide anatomic areas limit potential success of the procedure.
Carbon fiber table
To optimize the usefulness of the radiographic equipment, a nonmetallic, carbon-fiber surgical table is available for the interventional techniques.5 The preferred surgical operating table to accommodate such techniques should be thin but highly stable, and should provide complete clearance beneath a panning x-ray system. The telescoping pedestal should travel vertically from 28 to 48 inches, 20 degrees side-to side roll and 20 degrees Trendelenburg tilt (reverse and standard), which makes it ideal for a surgical operating room. Complete clearance is achieved beneath for unobstructed neck-to-toe imaging, and rapid horizontal panning is achieved with multiple position, height, tilt and roll adjustments.
Flat screens and monitors
The surgeons, assistant, anesthesiologist and nurse should all have views of all major imaging and monitoring sources. It is therefore suggested that display of all these sources should be available in all four quadrants of an integrated room. A total of 4-6 ceiling-mounted flat screens to be used as imaging tools for the procedures is necessary. Extreme care should be taken to ensure that these ceiling-mounted flat screens do not collide with operating lights. Monitors for the vital signs of the patient, with provision for systemic arterial monitoring, central venous monitoring, and continuous electrocardiographic surveillance, is imperative. A large 40-inch flat panel should be available as well as cameras (wall /or in-light).
Patient monitoring
The hybrid suite must be equipped for accurate patient monitoring during the procedure; for continuous electrocardiography, surveillance is imperative. Observation of urine output is also essential for cases involving the renal arteries and higher abdominal or thoracic aortic segments. Intra-arterial monitoring that includes precise measurement of pressure differentials is also important during performance of these procedures. Space for storage for the special procedure-related equipment such as stents, wires, balloons, and stent grafts should be available.
Fluoroscopy and CT imaging systems
The imaging quality is dominated by the quality of the fluoroscopy unit available. Available systems can be divided roughly into two categories: portable or fixed C-arm units. Fixed C-arm units could be floor- or ceiling-mounted. There are a number of fixed fluoroscopic units available with various modifications, depending on the manufacturer (e.g. Philips, GE, OEC, Siemens, Toshiba). The image quality of fixed systems is usually superior to portable systems, which can be explained by the focal spot sizes of fixed systems being significantly smaller than those of portable units. A smaller focal spot size means higher resolution through more line pairs per millimeter. Nevertheless, the latest portable C-arm systems are able to reach resolutions up to 2.5e3 line pairs per mm, values which only could be attained by fixed systems until recently. The monitor resolution of fixed systems differs from portable systems, with the monitors of fixed systems usually having twice the lines of resolution as the monitors of portable systems. Portable systems have a smaller generator in order to keep the system ‘‘practical’’ whilst fixed systems have a large remote generator, which provides more power with better tissue penetration and improved imaging quality. Currently, portable C-arm systems are able to provide sufficient quality for the majority of the standard procedures in cardiovascular surgery. However, the more complex procedures are best performed with a fixed unit.
Image acquisition and display
 Traditional fluoroscopy provides real-time, high-resolution, low-contrast images in two dimensions through the use of an image intensifier. The development of a flat-panel detector to replace the image intensifier has enabled fluoroscopy to transition into three dimensions, producing a CT-like image (Figure 3). The contrast resolution of CT is approximately 1 Hounsfield unit (HU), whereas the contrast resolution of a CT-like image is around 10 HU. CT fluoroscopy is not meant to replace diagnostic CT, but to be used as a tool that will supplement interventional procedures. The ability to acquire data in three dimensions during an intervention has led to the fusion of 3D datasets with the two-dimensional images displayed on typical monitors. In CT rotational angiography, found in the latest hybrid imaging systems, the C-arm is used to rapidly rotate, obtaining serial images of the area in question in a radial fashion. The 3D reconstruction can be registered with subsequent real-time fluoroscopic images and projected to offer the clinician the ability to work in three dimensions. The process by which the image is registered and displayed is the subject of considerable research efforts on the part of many imaging equipment manufacturers. Data can be rendered volumetrically and overlayed on the fluoroscopic image, making the anatomy much more identifiable. A fused two-dimensional/three-dimensional dataset can be created, or the information can be placed side by side. Further requirements of the suite’s imaging system are a processing unit, a workstation and a central image storage unit. The potential of any C-arm equals the weakest link of each of these last three elements. While performing a procedure, smooth and fast graphic abilities are a must. Using large-size, superb-quality images from a C-arm implies that a powerful processing unit is needed. The higher the image quality, the more working memory the processing unit needs. Images from a C-arm are stored in DICOM format files, which can then be used for biometric post processing, such as quantitative vessel analysis or three-dimensional reconstruction. The higher the quality of the images obtained from the C-arm, the larger the size of the files that have to be processed by the workstation. Advanced imaging using Dynamic 3D Roadmap (Philips) has significant clinical advantages for applications such as real-time catheter navigation and monitoring coil delivery. The image is dynamic, meaning the 3D roadmap remains displayed even if the C-arc projection, source-to-image distance or field of view size is changed. The 3D volume automatically follows the orientation of the C-arc in real time, so that users can choose the optimal projection view. This dynamic overlay ensures excellent positioning for catheter navigation during challenging interventions. The dynamic 3D image decreases the number of DSA acquisitions and fluoroscopy time for an examination. The user can also recall roadmap positions to reduce the need to re-mask. This reduces x-ray dose and contrast medium, which can reduce procedure costs. Dynamic 3D Roadmap provides live interventional catheter navigation.
Traditional fluoroscopy provides real-time, high-resolution, low-contrast images in two dimensions through the use of an image intensifier. The development of a flat-panel detector to replace the image intensifier has enabled fluoroscopy to transition into three dimensions, producing a CT-like image (Figure 3). The contrast resolution of CT is approximately 1 Hounsfield unit (HU), whereas the contrast resolution of a CT-like image is around 10 HU. CT fluoroscopy is not meant to replace diagnostic CT, but to be used as a tool that will supplement interventional procedures. The ability to acquire data in three dimensions during an intervention has led to the fusion of 3D datasets with the two-dimensional images displayed on typical monitors. In CT rotational angiography, found in the latest hybrid imaging systems, the C-arm is used to rapidly rotate, obtaining serial images of the area in question in a radial fashion. The 3D reconstruction can be registered with subsequent real-time fluoroscopic images and projected to offer the clinician the ability to work in three dimensions. The process by which the image is registered and displayed is the subject of considerable research efforts on the part of many imaging equipment manufacturers. Data can be rendered volumetrically and overlayed on the fluoroscopic image, making the anatomy much more identifiable. A fused two-dimensional/three-dimensional dataset can be created, or the information can be placed side by side. Further requirements of the suite’s imaging system are a processing unit, a workstation and a central image storage unit. The potential of any C-arm equals the weakest link of each of these last three elements. While performing a procedure, smooth and fast graphic abilities are a must. Using large-size, superb-quality images from a C-arm implies that a powerful processing unit is needed. The higher the image quality, the more working memory the processing unit needs. Images from a C-arm are stored in DICOM format files, which can then be used for biometric post processing, such as quantitative vessel analysis or three-dimensional reconstruction. The higher the quality of the images obtained from the C-arm, the larger the size of the files that have to be processed by the workstation. Advanced imaging using Dynamic 3D Roadmap (Philips) has significant clinical advantages for applications such as real-time catheter navigation and monitoring coil delivery. The image is dynamic, meaning the 3D roadmap remains displayed even if the C-arc projection, source-to-image distance or field of view size is changed. The 3D volume automatically follows the orientation of the C-arc in real time, so that users can choose the optimal projection view. This dynamic overlay ensures excellent positioning for catheter navigation during challenging interventions. The dynamic 3D image decreases the number of DSA acquisitions and fluoroscopy time for an examination. The user can also recall roadmap positions to reduce the need to re-mask. This reduces x-ray dose and contrast medium, which can reduce procedure costs. Dynamic 3D Roadmap provides live interventional catheter navigation.
Other imaging modalities
 Integration of other imaging modalities like intravascular ultrasound (IVUS) (Figure 4) permits more data acquisition.6,7 The explosion of the endovascular revolution with particular application to the aorta has placed new demands on accurate preoperative and intraoperative imaging to obtain accurate aortic measurements for endovascular stent grafting of the aorta. IVUS technology requires that the user be adequately versed in the process of performing the acquisition and interpretation of the images. In patients undergoing cardiac surgery, transesophageal echocardiography and epi-aortic ultrasound have been used to characterize the severity of atherosclerosis within the ascending aorta. Such information has been used to modify surgical technique, altering the location of cannula insertion, the position of aortic cross clamps and the placement of saphenous vein grafts, and reduce the risk of dislodging atheromatous debris.
Integration of other imaging modalities like intravascular ultrasound (IVUS) (Figure 4) permits more data acquisition.6,7 The explosion of the endovascular revolution with particular application to the aorta has placed new demands on accurate preoperative and intraoperative imaging to obtain accurate aortic measurements for endovascular stent grafting of the aorta. IVUS technology requires that the user be adequately versed in the process of performing the acquisition and interpretation of the images. In patients undergoing cardiac surgery, transesophageal echocardiography and epi-aortic ultrasound have been used to characterize the severity of atherosclerosis within the ascending aorta. Such information has been used to modify surgical technique, altering the location of cannula insertion, the position of aortic cross clamps and the placement of saphenous vein grafts, and reduce the risk of dislodging atheromatous debris.
Future perspectives
Wireless devices will become reality in the near future and would overcome the direct limitations now present due to wire connection points. In a wireless setting, the operating table, C-arm and other equipment can be rotated a full 360° (and beyond) at any location within the surgical/ endovascular suite. Wireless equipment would also save time in case one piece of equipment needs to be repaired. A broken piece of equipment can be temporarily removed from the interventional suite and be replaced by a spare. The cardiovascular surgeon or interventionist would not lose valuable operation time, and patients would not need to be put on hold. The technical team would not have to wait for spare parts or specialized tools for a certain repair because the broken piece could easily be shipped to a central repair point. Moreover, this approach would save time and costs related to the mobility of a highly-specialized technical team. Integration of robotic and navigational techniques into clinical practice may lead to improved catheter accuracy, stability, and safety in comparison with conventional techniques, while minimizing radiation exposure. By maximizing the use of existing technologies while developing new approaches to treating these challenging cases, we hope that these would lead to the improvement of overall clinical outcomes and further reduce the mortality and morbidity rates associated with managing the cardiovascular patient. It is hoped that as these new fields develop and increase experience with these new hybrid methods, we may well be able to maximize the applicability of minimally invasive endovascular and hybrid technology to treat a larger cohort of patients with cardiovascular disease.8
Dr. Kpodonu is board certified in general surgery, cardiothoracic surgery and endovascular medicine. He can be contacted at Jacques.Kpodonu@hoag.org.
Disclosure: Dr. Kpodonu reports no conflicts of interest regarding the content herein.
References
- Eliason JL, Guzman RJ, Passman MA, Naslund TC. Infected endovascular graft secondary to coil embolization of endoleak: a demonstration of the importance of operative sterility. Ann Vasc Surg 2002;16:562e565.
- Nichols RL. The operating room. In: Bennett JV, Brachman PS, Sanford JP, Eds. Hospital infections. 3rd ed. Boston, MA: Little, Brown; 1992: 461-467.
- Goel VR, Ambekar A, Greenburg RK. Multimodality imaging and image-guided surgery. Advances in imaging provide physicians with the confidence to confront procedures previously thought impossible. Endovascular Today March 2008;67-70.
- Mansour MA. Endovascular and minimally invasive vascular surgery. The new operating environment. Surg Clin North Am 1999;79:477e487.
- Fillinger MF, Weaver JB. Imaging equipment and techniques for optimal intraoperative imaging during endovascular interventions. Semin Vasc Surg 1999;12:315e326.
- Kpodonu J, Ramaiah VG, Diethrich EB. Intravascular ultrasound imaging as applied to the aorta: a new tool for the cardiovascular surgeon. Ann Thorac Surg 2008 Oct; 86(4):1391-1398.
- Kpodonu J, Raney AA. The cardiovascular hybrid room: a key component for multimodality for hybrid interventions and image guided surgery in the emerging specialty of cardiovascular hybrid surgery. Interact Cardiovasc Thorac Surg 2009 Oct; 9(4):688-692.
- Kpodonu J. Hybrid cardiovascular suite: the operating room of the future. J Card Surg 2010 Nov; 25(6):704-709.










