Bleeding and Vascular Complications at the Femoral Access Site Following Percutaneous Coronary Intervention: An Evaluation of Hemostasis Strategies
Abstract: Background. Previous research found at least one vascular closure device (VCD) to be associated with excess vascular complications, compared to manual compression (MC) controls, following cardiac catheterization. Since that time, several more VCDs have been approved by the Food and Drug Administration (FDA). This research evaluates the safety profiles of current frequently used VCDs and other hemostasis strategies. Methods. Of 1089 sites that submitted data to the CathPCI Registry from 2005 through the second quarter of 2009, a total of 1,819,611 percutaneous coronary intervention (PCI) procedures performed via femoral access site were analyzed. Assessed outcomes included bleeding, femoral artery occlusion, embolization, artery dissection, pseudoaneurysm, and arteriovenous fistula. Seven types of hemostasis strategy were evaluated for rate of “any bleeding or vascular complication” compared to MC controls, using hierarchical multiple logistic regression analysis, controlling for demographic factors, type of hemostasis, several indices of co-morbidity, and other potential confounding variables. Rates for different types of hemostasis strategy were plotted over time, using linear regression analysis. Results. Four of the VCDs and hemostasis patches demonstrated significantly lower bleeding or vascular complication rates than MC controls: Angio-Seal (odds ratio [OR], 0.68; 95% confidence interval [CI], 0.65-0.70); Perclose (OR, 0.54; CI, 0.51-0.57); StarClose (OR, 0.77; CI, 0.72-0.82); Boomerang Closure Wire (OR, 0.63; CI, 0.53-0.75); and hemostasis patches (OR, 0.70; CI, 0.67-0.74). All types of hemostasis strategy, including MC, exhibited reduced complication rates over time. All trends were statistically significant except one. Conclusions. This large, nationally representative observational study demonstrated better safety profiles for most of the frequently used VCDs, compared to MC controls.
J INVASIVE CARDIOL 2012;24(7):328-334
Key words: hemostasis patch, mechanical compression, vascular closure device
___________________________________________________
Bleeding and vascular complications were the most common non-cardiac, procedure-related adverse outcomes of the estimated 1,178,000 percutaneous coronary interventions (PCIs) performed in 2007.1,2 While it is not surprising that adverse vascular events are associated with a procedure that begins via puncture of an artery, the number and type of local vascular complications, and the clinical outcomes associated with them (increased morbidity, mortality, and length of stay in the hospital), underscore the importance of continuing surveillance by the Food and Drug Administration (FDA).
Clinicians who performed PCIs in the early years of the procedure achieved hemostasis after femoral sheath removal via manual and/or mechanical compression approaches. These hemostasis strategies required that patients remain immobilized for extended periods of time (up to 8 hours after a procedure). This approach created substantial discomfort and extended hospital stays. Alternative methods of achieving hemostasis were introduced into cardiac catheterization laboratories approximately 20 years ago. Loosely termed vascular closure devices (VCDs), these alternatives typically included sutures, sealants, clips, and arterial compression mechanisms, and offered clinicians an alternative to manual and mechanical compression. Since the inception of these devices, the federal government has required that they receive premarketing approval from the FDA, as well as undergo postmarketing surveillance and safety assessments. The FDA has approved these devices for the purpose of decreasing the amount of time to achieve hemostasis, which thereby allows patients to ambulate earlier.3
Between 1996 and 2000, nearly 2000 reports of serious adverse events and 36 deaths associated with the use of VCDs were received by the FDA through its routine surveillance system, with a large proportion of these events occurring in women.4 Because of its concern about these reports, the FDA collaborated with the American College of Cardiology (ACC) and its National Cardiovascular Data Registry (NCDR) to analyze closure-device related adverse events. Analysis indicated that a higher rate of vascular complications was associated with one particular device,5 which was subsequently, and voluntarily, removed from the market by its manufacturer. This experience underscored the value of investigating real-world methods of hemostasis via analysis of data collected in observational registries such as those within the NCDR. Since that study, the FDA has approved more closure devices, and is again collaborating with the NCDR to evaluate safety profiles of the most frequently used closure devices, and compare their safety profiles to manual and mechanical compression.
Methods
Data source. The data used in this study were obtained from the NCDR CathPCI Registry. This registry is co-sponsored by the American College of Cardiology (ACC) and the Society for Cardiovascular Angiography and Interventions (SCAI). Data analyzed in this study utilized cath lab module v3, which included more than 200 core data elements needed for measuring the clinical management and outcomes of patients undergoing diagnostic cardiac catheterizations and PCIs.6 Although it is voluntary, several states and health plans require participation in the NCDR CathPCI Registry to fulfill state or performance recognition reporting requirements. As of June 2009, over 1200 institutions had joined the CathPCI Registry. This study included data from 1089 sites, and from 1,861,566 patients who received PCI and were discharged between January 1, 2005 and June 30, 2009.
Outcomes and definitions. Only outcomes that occurred during the hospital stay were included in this analysis. Data were analyzed according to 3 outcome categories:
Bleeding complication: Blood loss at the site of arterial or venous access, or due to perforation of a traversed artery or vein requiring transfusion and/or prolonging the hospital stay, and/or causing a drop in hemoglobin of >3.0 g/dL. Bleeding attributable to the vascular site could be retroperitoneal (retroperitoneal bleeding), a local hematoma >10 cm with femoral access, >2 cm with radial access, or >5 cm with brachial access (hematoma bleeding), or external (entry site bleeding).
Vascular complication: This category included the presence of any one of the following vascular complications pertaining to the percutaneous access site: occlusion, defined as total obstruction of the artery by thrombus, usually at the site of access requiring surgical repair; embolization, defined as loss of distal pulse, pain and/or discoloration (especially the toes); dissection, defined as a disruption of an arterial wall resulting in splitting and separation of the intimal (subintimal) layers; pseudoaneurysm, defined as the occurrence of a disruption and dilation of the arterial wall without identification of the arterial wall layers at the site of the catheter entry demonstrated by arteriography or ultrasound; or AV fistula, defined as a connection between the access artery and the accompanying vein demonstrated by arteriography or ultrasound and most often characterized by a continuous bruit.
Bleeding or vascular complication: Either one or the other, or both.
Inclusion/exclusion criteria. Catheterization laboratory discharges involving PCI for the first quarter of 2005 through the second quarter of 2009 were included in this analysis. Excluded from the analysis were: (1) any subject for whom information was not complete with regard to type of hemostasis strategy or for whom the strategy used was characterized by less than 5000 uses during the period of the study; (2) any subject who was discharged on the same day of admission who didn’t die prior to discharge; and (3) data submissions were excluded if they failed to pass data quality standards.
Statistical analysis. Hierarchical multiple logistic regression analyses were performed separately using “bleeding complications,” “vascular complications,” and “bleeding or vascular complications” as the dependent variables. In this model, two-level data of patients within hospitals were considered and random effect of patient-level intercept was used for the clustering of patients among hospitals.
Independent variables were selected based on clinical meaning and medical publications based on our previous experience, which included age, gender, race (white vs non-white vs other), type of hemostasis strategy, body mass index (BMI), several indices of co-morbidity (New York Heart Association [NYHA] classification, presence of diabetes, hypertension, peripheral vascular disease, left main coronary artery stenosis, shock, acute renal failure, history of congestive heart failure, and previous recent PCI), status of procedure (routine, urgent, emergency, or salvage), use of IABP, use of anticoagulants or anti-platelet agents during procedure (aspirin, IIb/IIIa inhibitors, thrombin inhibitors, thrombolytics, low molecular weight heparin, and unfractionated heparin, each assessed individually), and number of annual admissions for PCI. In the hierarchial logistic models, two-level data of patients within hospitals were considered. Missing values were rare, and imputed as the median values for continuous variables and as the most common category for categorical variables. Odds ratios and P-values were calculated for all independent variables with respect to the clinical endpoints.
The “type of hemostasis strategy” was characterized in 2 different ways. The first was by 1 of the following 8 “strategy groups:” manual compression (MC); mechanical compression devices (MCD); hemostasis patches; and 1 of 5 VCDs, including Perclose (Abbott Vascular); Angio-Seal (St Jude Medical, Inc); Boomerang Closure Wire (Cardiva Medical, Inc); Mynx (AccessClosure, Inc); and StarClose (Abbott Vascular). The other was by more specific identification of VCDs within some of the larger groupings, as follows: Perclose (A-T and ProGlide); Angio-Seal (plain, VIP, STS, and STS PLUS); StarClose (vascular closure system and SE); and patches (Clo-Sur pad, Chito-Seal, Syvek Patch, D-stat, and Neptune Patch). Type of hemostasis strategy was defined according to the first strategy used in each subject in each visit. For that purpose, strategies were categorized into 3 hierarchical levels (Level 1 = manual or mechanical compression; Level 2 = any patch; and Level 3 =any VCD), whereby “first strategy used” was defined as the first strategy used in the highest categorical level for a particular procedure. The independent effect of the type of hemostasis strategy on clinical outcome was evaluated using the hierarchical logistic regression models. Adjusted ORs and P-values for each combination of clinical outcome and type of hemostasis strategy were reported, using MC as the reference group.
In addition, the trend in “bleeding or vascular complications” was plotted by quarter for each device group, beginning with the quarter in which the device was used in at least 10 cath lab visits. Linear regression, using the rate for each quarter as the unit of analysis, was then used to assess for statistically significant trends.
The hemostasis strategy comparison analyses for this study included only femoral access site subjects. Other access sites (including brachial, radial, and “other”) were added in multivariate analysis in order to compare complication rates for the different access sites.
Data quality. The CathPCI Registry has an established Data Quality Program that includes consistency in the definition of data elements, data manager training, data quality assessments with data submissions (data quality reports), and a Data Audit Program. The data quality report (DQR) assesses the completeness of data submitted by participating facilities. Facilities must achieve a certain percentage of completeness of specific data elements identified to be included in the data warehouse for analysis. For example, completeness thresholds for inclusion range between 90% and 100%. The DQR allows facilities the opportunity to correct errors and resubmit data for review and acceptance in the data warehouse. All data for this analysis passed the DQR completeness thresholds. The Data Audit Program consists of annual on-site review and data abstraction at 25 randomly selected sites. At each site, a random sample of 25 patient records is reviewed and analyzed for accuracy.
Results
Sample sizes. There were 2,063,515 PCI procedures included in the registry from the first quarter of 2005 through the second quarter of 2009. Of these, 201,949 procedures (9.8%) were excluded from analysis for one of the above-noted considerations. That left 1,861,566 PCI procedures that were included in the study. Of these, there were 1,819,611 PCI procedures with femoral access (97.7%), 27,402 with radial access (1.5%), 6878 with brachial access (0.4%), and 7675 procedures with other access, missing values or with no arterial catheter (0.4%).
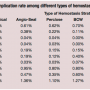 Use of the different types of hemostasis strategy varied substantially in the studied population: MC: 723,859 (38.9%); mechanical compression devices (MCD): 185,039 (9.9%); Angio-Seal: 534,556 (28.7%); Perclose: 155,279 (8.3%); StarClose: 81,242 (4.4%); Mynx: 23,690 (1.3%); Boomerang Closure Wire: 10,950 (0.6%); and patches: 146,951 (7.9%).
Use of the different types of hemostasis strategy varied substantially in the studied population: MC: 723,859 (38.9%); mechanical compression devices (MCD): 185,039 (9.9%); Angio-Seal: 534,556 (28.7%); Perclose: 155,279 (8.3%); StarClose: 81,242 (4.4%); Mynx: 23,690 (1.3%); Boomerang Closure Wire: 10,950 (0.6%); and patches: 146,951 (7.9%).
Univariate analysis. Table 1 shows complication rates in univariate analysis for every outcome among different strategy groups. Every VCD was associated with lower complication rates than MC for every clinical outcome except for retroperitoneal bleed. Hemostasis patches demonstrated complication rates that were higher than the VCDs but lower than MC or MCD.
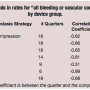 Trend analysis. Table 2 shows the trend analysis by quarter (beginning with the first quarter in which there were 10 or more PCIs using that hemostasis strategy) for “bleeding or vascular complication” rates over time, for each of the 8 hemostasis strategy groups.
Trend analysis. Table 2 shows the trend analysis by quarter (beginning with the first quarter in which there were 10 or more PCIs using that hemostasis strategy) for “bleeding or vascular complication” rates over time, for each of the 8 hemostasis strategy groups.
All 8 types of hemostasis were associated with decreasing rates over time, as demonstrated by correlation coefficients between the corresponding rates and time period ranging from -0.51 to -0.92. All trends were statistically significant at the P<.02 level, except for Mynx, which was not statistically significant at the P<.05 level.
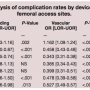 Multivariate analysis. Multivariate analyses involved only PCIs with femoral artery access sites, except for the analysis that compared rates by access site. Table 3 shows the adjusted ORs (with MC as the reference group) for each of the other 7 hemostasis strategy groups for the 3 major outcomes (“bleeding complications,” “vascular complications,” and “bleeding or vascular complications”).
Multivariate analysis. Multivariate analyses involved only PCIs with femoral artery access sites, except for the analysis that compared rates by access site. Table 3 shows the adjusted ORs (with MC as the reference group) for each of the other 7 hemostasis strategy groups for the 3 major outcomes (“bleeding complications,” “vascular complications,” and “bleeding or vascular complications”).
All of the hemostasis strategies performed better compared to MC, except for mechanical compression devices and Mynx. The other hemostasis strategy groups all demonstrated significantly low odds of “bleeding or vascular complications” compared to MC, including Angio-Seal (OR, 0.68; CI, 0.65-0.70; P<.001), Perclose (OR, 0.54; CI, 0.51-0.57; P<.001), StarClose (OR, 0.77; CI, 0.72-0.82; P<.001), Boomerang Closure Wire (OR, 0.63; CI, 0.53-0.75; P<.001), and patches (OR, 0.70; CI, 0.67-0.74; P<.001). Mechanical compression demonstrated significantly higher odds of “bleeding or vascular complications” than MC (OR, 1.15; CI, 1.10-1.20; P<.001), and there was no significant difference in “bleeding or vascular complications” between MC and Mynx.
All hemostasis strategy groups demonstrated significantly lower odds of vascular complications than MC, except for mechanical compression devices, which demonstrated a significantly higher rate. Perclose, Angio-Seal, and patches also demonstrated significantly lower odds of bleeding complications than MC. There were no significant differences in bleeding complications compared to MC for StarClose and Boomerang. Mechanical compression and Mynx demonstrated significantly higher odds of bleeding complications than MC.
Within the Perclose device group, both specific devices (A-T and ProGlide) were associated with significantly lower odds of “bleeding or vascular complications” than MC. However, when compared to MC, Perclose ProGlide (OR, 0.51; CI, 0.48-0.55) was associated with a lower OR than Perclose A-T (OR, 0.64; CI, 0.58-0.71). Within the StarClose group, both devices were associated with significantly lower odds of “bleeding or vascular complications” than MC. However, StarClose SE (OR, 0.57; CI, 0.44-0.73) was associated with a lower OR than StarClose Vascular Closure System (OR, 0.79; CI, 0.74-0.84) when compared to MC. Within the Angio-Seal group, all the specific devices were associated with significantly lower odds of “bleeding or vascular complications” than MC. However, Angio-Seal VIP (OR, 0.63; CI, 0.60-0.66) and Angio-Seal STS Plus (OR, 0.61; CI, 0.57-0.65) were associated with significantly lower odds than the other two (Angio-Seal STS and plain Angio-Seal). Although the hemostasis patches, as a group, were associated with significantly lower odds of “bleeding or vascular complications” than MC, two patches were not: D-stat (Vascular Solutions) demonstrated by far the lowest odds of “bleeding or vascular complications” (OR, 0.43; CI, 0.39-0.48). Clo-Sur Pad (Scion Cardio-Vascular; OR, 1.04; CI, 0.87-1.26) and Neptune Patch (TZ Medical, Inc; OR, 0.91; CI, 0.79-1.04) were not associated with lower complication odds than MC.
 Table 4 shows the ORs, with CIs and P-values for each of the co-variables that demonstrated significant ORs in patients with femoral artery access sites.
Table 4 shows the ORs, with CIs and P-values for each of the co-variables that demonstrated significant ORs in patients with femoral artery access sites.
Co-variates that were associated with an increased risk of “bleeding or vascular complications” included, age, female sex, body mass index, renal failure, peripheral vascular disease, hypertension, congestive heart failure, New York Heart Association (NYHA) class IV, acute myocardial infarction, recent PCI, history of congestive heart failure, use of IABP during the procedure, PCI status of emergency, urgent or salvage, and use of IIb/IIIa inhibitors, thrombolytics, low molecular weight heparin or unfractionated heparin during the procedure. Co-variables that were associated with a decreased risk of “bleeding or vascular complications” included black race, diabetes, cardiogenic shock, left main coronary artery disease, and the use of aspirin or thrombin inhibitors (which included bivalirudin, argatroban, or lepirudin-rDNA) during the procedure.
In multivariate analysis, a radial artery access site was associated with far lower odds of “bleeding or vascular complications” compared to femoral access site (OR, 0.33; CI, 0.29-0.39; P<.001). Both bleeding and vascular complications were substantially lower for radial access site patients than for femoral access site patients. A brachial access site was associated with far higher odds of “bleeding or vascular complications” compared to femoral access site (OR, 2.41; CI, 2.15-2.69; P<.001).
Missing values for variables used in the multivariate regression equations were very low. The highest rate was for race, for which there were only 2872 missing values (0.15% of the total sample). The highest number of missing values for a co-morbid condition was NYHA class, for which there were only 415 missing values (only 0.02% of the total sample).
Discussion
Historical findings. Our findings are consistent with the body of literature that examines VCD use specifically associated with PCI. A majority of this literature consists of small, uncontrolled studies that report a wide range of safety and efficacy endpoints according to varied clinical definitions.
Most recent studies show evidence that VCDs are associated with safety profiles that are not significantly different than manual compression,7-10 including several randomized controlled trials that compare specific brands to each other and against MC controls.11-15 Several other studies present evidence that VCDs are associated with decreased risk of bleeding and/or vascular complications,16-19 including an analysis by Marso et al, which used data from the CathPCI Registry to examine periprocedural bleeding complications in 1,522,935 patients.20 That study used data from the same registry as the FDA-ACC studies that assessed patients who underwent PCI from Jan 1, 2004 through September 30, 2008. It found that VCD use was associated with a significant reduction in bleeding events relative to MC (OR, 0.77; CI, 0.73-0.80). Another large registry capturing 45,987 patients undergoing PCI from 2002-200721 found VCD use to be associated with a significant reduction in “bleeding and vascular complications” for both men and women compared to non-VCD use (OR, 0.75; P<.007 and OR, 0.72 and P=.0002, respectively). Although these large, representative registries did not report their findings by device type, the results regarding local vascular complications of VCDs as a whole are very similar to the FDA-ACC findings noted above.
An investigation of predictors of retroperitoneal hemorrhage following PCI in 112,430 patients in the Blue Cross Blue Shield of Michigan Cardiovascular Consortium (BMC2) Registry found that VCDs, and in particular Angio-Seal, were more frequently used in patients who developed retroperitoneal hemorrhage than in those who did not. The association was significant for Angio-Seal (OR, 1.68; P<.0001), whereas it was non-significant for Perclose (OR, 1.29; P=.13).22 Our study found that retroperitoneal bleed occurred in 0.38% of patients who received Angio-Seal, compared to 0.22% who received Perclose, and 0.26% of the MC controls — thus confirming the BMC2 study with regard to the relatively high risk of Angio-Seal (and some other VCDs) for retroperitoneal bleed.
Four separate meta-analyses reflect trials related to the early generations of VCDs and demonstrate a considerable amount of overlap23-26 with each other. Two of these meta-analyses represent outcomes from the initial clinical trials of VCDs,24,25 and another one was also influenced by the results of these trials.26 The major finding from these meta-analyses was that VCDs generally performed as well or better than MC controls, but that there was strong evidence to show Vasoseal performed substantially worse than either Perclose or Angio-Seal with respect to the three most frequently reported outcomes — hematoma, bleeding, and pseudoaneurysm.
Koreny et al’s meta-analysis,24 which included almost 4000 patients across 30 clinical trials, suggested that when results were limited to trials that used intention-to-treat analysis, VCDs were associated with a higher risk of hematoma and pseudoaneurysm. However, there was no separate analysis of diagnostic and interventional procedures, and the results must be interpreted with caution due to the variability of study reporting and endpoint definitions.
For example, Nikolsky et al25 catalogues the wide range of hematoma definitions described in study methodologies and notes at least a dozen different descriptions in the 30 trials included in their analysis. That study attempted to address the heterogeneity of study results by performing separate analyses of diagnostic and PCI procedures, as well as by device type. With respect to major vascular complications, the authors found that Angio-Seal and Perclose were similar to MC for complications associated with PCI.
Biancari et al’s meta-analysis reflected relatively more recent randomized studies (about one-third of the included studies were published after the previous three meta-analyses), but is generally consistent with the other three meta-analyses.26 That study found some evidence for an increased risk of groin infection, arterial complications resulting in arterial stenosis, and lower limb ischemia, as well as the need for vascular surgery for repair of arterial complications after the use of VCDs. These findings appear to be more evident in patients undergoing PCI than diagnostic procedures, although the authors note that they may be significantly biased by poor methodological quality of available studies.
Study strengths and limitations. This study is characterized by several advantages over most other studies performed to assess the relative safety profiles of VCDs compared to MC. It is by far the largest study to date, including 1,861,566 cardiac catheterizations from a broadly representative sample of 1089 different institutions throughout the United States. As such, the power of the study was great enough to assess comparisons between 5 of the most commonly used VCDs versus MC controls with very narrow CIs. It utilized standard definitions and meticulous quality control procedures. It controlled for the potential confounding effects of numerous variables known to influence local vascular complication rates following cardiac catheterization. The fact that this is an observational study means that the results are not affected by the kinds of stringent conditions that typically characterize clinical trials, but rather the results are reflective of real-world settings.
It is, however, possible that unknown and unmeasured variables could have exerted a confounding effect that was undetected by this study. For example, physicians may have been reluctant to use VCDs in certain high-risk situations, such as where an injury occurred to the vessel wall during the procedure, when a groin hematoma occurred during the catheterization, or when a “predeployment” femoral angiogram demonstrated the puncture site to present a risk that was thought to contraindicate the use of a VCD. If so, these considerations would have biased the study results against MC. This type of situation probably accounted, at least in part, for the apparent protective effect of VCDs. On the other hand, a very high puncture (with consequent high risk of retroperitoneal bleed) may have encouraged the use of a VCD because of the difficulty of MC in those cases. Another unmeasured confounding variable could have been sheath size, which is known to be associated with a high risk of local vascular complications,27 and which could also have been correlated with a decision not to use a VCD, thus biasing the study results in favor of VCDs.
It should also be noted that the bleeding and vascular complication rates pertaining to each hemostasis strategy in this study are the mean rates over the time period of the study. As noted in Table 2, these rates decreased over time for each hemostasis strategy evaluated in this study. Therefore, the current rates and comparisons between the VCD subjects and MC controls differ somewhat from the statistics described in this study, to the extent that the magnitude of the decrease in rates differed between the VCD subjects and the controls.
Conclusion
Overall, the medical literature on this issue is highly consistent with the findings of this study, with VCDs generally demonstrating good performance against MC controls across a wide range of treatment groups and clinical outcomes (with the exception of retroperitoneal bleed, which was positively associated with VCD use). Most of these studies were very small compared to the FDA-ACC study, and consequently most of them demonstrated no statistically significant clinical differences between MC versus VCDs as a group, let alone specific VCDs. But there were several studies in the medical literature that demonstrated superior safety profiles for the VCDs compared to MC controls, and those studies tended to analyze data accrued over more recent time periods. That is consistent with our analyses, which found improving safety profiles over time for all of the VCDs. There are three possible explanations for these improving safety profiles over time: (1) over time the VCDs have become smaller and less cumbersome and easier to use; (2) health care professionals using the devices have become more adept at using them as they gain more experience with them; and (3) case selection may have improved over time.
Acknowledgments. We would like to acknowledge the Office of Women’s Health at the FDA for providing the funding for this research.
References
- Marso SP, Amin AP, House JA, et al; on behalf of the National Cardiovascular Data Registry. Association between use of bleeding avoidance strategies and risk of periprocedural bleeding among patients undergoing percutaneous coronary intervention. JAMA. 2010;303(21):2156-2164.
- Heart Disease and Stroke Statistics — 2011 Update. American Heart Association, 2011.
- Tavris DR, Gallauresi BA, Dey S, Brindis R, Mitchel K. Risk of local adverse events by gender following cardiac catheterization. Pharmacoepidemiol Drug Saf. 2007;16(2):125-131.
- United States Food and Drug Administration (US FDA). Manufacturer and user facility device experience; MAUDE Database, 2001: Accessed at www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfmaude/TextResults.cfm
- Tavris DR, Dey S, Gallauresi B, et al. Risk of local adverse events following cardiac catheterization by hemostasis device use — phase II. J Invasive Cardiol. 2005;17(12):644-650.
- An initiative of the American College of Cardiology Foundation, the NCDR, National Cardiovascular Data Registry, is a comprehensive, outcomes-based suite of registries focused on quality improvement. www.ncdr.com.
- Applegate RJ, Sacrinty MT, Kutcher MA, et al. Propensity score analysis of vascular complications after diagnostic cardiac catheterization and percutaneous coronary intervention 1998-2003. Catheter Cardiovasc Interv. 2006;67(4):556-562.
- Applegate RJ, Sacrinty M, Kutcher MA, et al. Vascular complications with newer generations of Angio-Seal vascular closure devices. J Interv Cardiol. 2006;19(1):67-74.
- Applegate RJ, Sacrinty MT, Kutcher MA, et al. Propensity score analysis of vascular complications after diagnostic cardiac catheterization and percutaneous coronary intervention using thrombin hemostatic patch-facilitated manual compression. J Invasive Cardiol. 2007;19(4):164-170.
- Sulzbach-Hoke LM, Ratcliffe SJ, Kimmel SE, et al. Predictors of complications following sheath removal with percutaneous coronary intervention. J Cardiovasc Nurs. 2010;25(3):E1-E8.
- Legrand V, Doneux P, Martinez C, et al. Femoral access management: comparison between two different vascular closure devices after percutaneous coronary intervention. Acta Cardiol. 2005;60(5):482-488.
- Hermiller JB, Simonton C, Hinohara T, et al. The StarClose Vascular Closure System: interventional results from the CLIP study. Catheter Cardiovasc Interv. 2006;68(5):677-683.
- Martin JL, Pratsos A, Magargee E, et al. A randomized trial comparing compression, Perclose Proglide and Angio-Seal VIP for arterial closure following percutaneous coronary intervention: the CAP trial. Catheter Cardiovasc Interv. 2008;71(1):1-5.
- Deuling JH, Vermeulen RP, Anthonio RA, et al. Closure of the femoral artery after cardiac catheterization: a comparison of Angio-Seal, StarClose, and manual compression. Catheter Cardiovasc Interv. 2008;71(4):518-523.
- Wong SC, Bachinsky W, Cambier P, et al; ECLIPSE Trial Investigators. A randomized comparison of a novel bioabsorbable vascular closure device versus manual compression in the achievement of hemostasis after percutaneous femoral procedures: the ECLIPSE (Ensure’s Vascular Closure Device Speeds Hemostasis Trial). JACC Cardiovasc Interv. 2009;2(8):785-793.
- Arora N, Matheny ME, Sepke C, Resnic FS. A propensity analysis of the risk of vascular complications after cardiac catheterization procedures with the use of vascular closure devices. Am Heart J. 2007;153(4):606-611.
- Castillo-Sang M, Tsang AW, Almaroof B, et al. Femoral artery complications after cardiac catheterization: a study of patient profile. Ann Vasc Surg. 2010;24(3):328-335.
- Sanborn TA, Ebrahimi R, Manoukian SV, et al. Impact of femoral vascular closure devices and antithrombotic therapy on access site bleeding in acute coronary syndromes: the Acute Catheterization and Urgent Intervention Triage Strategy (ACUITY) trial. Circ Cardiovasc Interv. 2010;3(1):57-62.
- Iqtidar AF, Li D, Mather J, McKay RG. Propensity matched analysis of bleeding and vascular complications associated with vascular closure devices vs standard manual compression following percutaneous coronary intervention. Conn Med. 2011;75(1):5-10.
- Marso SP, Amin AP, House JA, et al; National Cardiovascular Data Registry. Association between use of bleeding avoidance strategies and risk of periprocedural bleeding among patients undergoing percutaneous coronary intervention. JAMA. 2010;303(21):2156-2164.
- Ahmed B, Piper WD, Malenka D, et al. Significantly improved vascular complications among women undergoing percutaneous coronary intervention: a report from the Northern New England Percutaneous Coronary Intervention Registry. Circ Cardiovasc Interv. 2009;2(5):423-429.
- Trimarchi S, Smith DE, Share D, et al; BMC2 Registry. Retroperitoneal hematoma after percutaneous coronary intervention: prevalence, risk factors, management, outcomes, and predictors of mortality: a report from the BMC2 (Blue Cross Blue Shield of Michigan Cardiovascular Consortium) registry. JACC Cardiovasc Interv. 2010;3(8):845-850.
- Vaitkus PT. A meta-analysis of percutaneous vascular closure devices after diagnostic catheterization and percutaneous coronary intervention. J Invasive Cardiol. 2004;16(5):243-246.
- Koreny M, Riedmuller E, Nikfardjam M, et al. Arterial puncture closing devices compared with standard manual compression after cardiac catheterization — systematic review and meta-analysis. JAMA. 2004;291(3):350-357.
- Nikolsky E, Mehran R, Halkin A, et al. Vascular complications associated with arteriotomy closure devices in patients undergoing percutaneous coronary procedures: a meta-analysis. J Am Coll Cardiol. 2004;44(6):1200-1209.
- Biancari F, D’Andrea V, Di Marco C, et al. Meta-analysis of randomized trials on the efficacy of vascular closure devices after diagnostic angiography and angioplasty. Am Heart J. 2010;159(4):518-531.
- Tavris DR, Dey S, Gallauresi B, et al. Risk of local adverse events following cardiac catheterization by hemostasis device use — phase II. J Invasive Cardiol. 2005;17(12): 644-650.
___________________________________________________
From the 1US Food and Drug Administration (FDA), Silver Spring, Maryland, 2Yale University, New Haven, Connecticut, 3University of Colorado, Boulder, Colorado, 4Brigham and Women’s Hospital, Boston, Massachusetts, and 5the American College of Cardiology, Bethesda, Maryland.
Disclaimer: Opinions expressed in this article are those of the authors only, not the FDA.
Disclosures: The authors have completed and returned the ICMJE Form for Disclosure of Potential Conflicts of Interest. Dr Curtis provides expert testimony for the Piedmont Liability Trust, holds a grant from the ACC Foundation, NIH/NHLBI Center for Medicare and Medicaid Services, and owns stock in Medtronic, Inc. Dr Resnic is a consultant for St Jude Medical and Medtronic, Inc and holds a Research Registry support grant from St Jude Medical. Dr Fitzgerald received payment in the form of a contract to perform the analysis and is a board member of the ACC Foundation. The other authors report no disclosures.
Manuscript submitted December 8, 2011, provisional acceptance given February 14, 2012, final version accepted March 16, 2012.
Address for correspondence: Dale R. Tavris, MD, MPH, Division of Epidemiology, Center for Devices and Radiological Health, US Food and Drug Administration. Email: dale.tavris@fda.hhs.gov.










