Ask the Clinical Instructor
 Todd is the Cardiology Manager for Memorial Hermann Southeast in Houston, Texas. He also teaches an online RCIS Review course for Spokane Community College, in Spokane, Washington, and regularly presents with RCIS Review Courses.
Todd is the Cardiology Manager for Memorial Hermann Southeast in Houston, Texas. He also teaches an online RCIS Review course for Spokane Community College, in Spokane, Washington, and regularly presents with RCIS Review Courses.
“We are now being deluged with information from vendors about purchasing equipment for pressure wires. What is this all about? Why will it help us?”
— Submission to tginapp@rcisreview.com
I will answer the second part of your question — “why will it help us?” — first. You may hear more and more about pressure wires now that the FAME II (FFR vs Angiography in Multivessel Evaluation) study is being discussed. FAME II was a follow-up to the initial FAME study published in 2009 (https://www.famestudy.com). We recommend readers refer to articles in this issue from Drs. Kern and Fearon to learn more about these trials.
Now, on to the first part of your question: “What is this all about?” In the November 2008 issue of CLD, available at www.cathlabdigest.com, we discussed the differences between fractional flow reserve (FFR) and intravascular ultrasound (IVUS). We will review some of the important parts of FFR from that article, and expand and explain some of the principles and process behind FFR.
FFR is a tool that evaluates the physiology of blood flow through an artery. It can directly determine the quantity of blood flow past a questionable lesion (more on that later).
While coronary angiography can clearly identify many lesions, there are also some limitations to seeing lesions on angiography:
- It’s only a “lumenography.” In other words, we can only see where the contrast is traveling. We cannot see the entire vessel structure and how well that contrast flows downstream.
- With diffuse disease, we do not know which vessels may actually be causing the symptoms, or, for vessels with multiple disease locations, which lesion(s) may actually be causing the ischemic conditions.
- In the case of an intermediate lesion (40-70% on angiography), we still can’t tell on angio if this lesion is causing symptoms, and whether it needs to be fixed or not.
Fractional flow reserve (FFR) is, simply put, a “stress test on the table.” Medication is administered (adenosine is recommended) to dilate the microvasculature to obtain the maximum blood flow/perfusion possible (hyperemia) distal to the lesion.
 A pressure reading is obtained proximal and distal to the lesion, and a gradient is established to determine any flow restriction during exercise. This is accomplished by simultaneously obtaining the proximal pressure through the guiding catheter, and the distal pressure by the pressure wire. What is happening is that maximum achievable blood flow in the stenotic coronary artery is divided by the maximum blood flow in the same artery without stenosis to determine the restriction of flow past the blockage.1
A pressure reading is obtained proximal and distal to the lesion, and a gradient is established to determine any flow restriction during exercise. This is accomplished by simultaneously obtaining the proximal pressure through the guiding catheter, and the distal pressure by the pressure wire. What is happening is that maximum achievable blood flow in the stenotic coronary artery is divided by the maximum blood flow in the same artery without stenosis to determine the restriction of flow past the blockage.1
In the absence of epicardial coronary artery disease in a vessel, the FFR of 1.0 is normal, but coronary arteries generally show a FFR > 0.94. FFR findings and their generally recommended course of treatment can be seen in Table 1.2,4
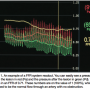 While your FFR system may be different, most will give you a readout similar to what can be seen in Figure 1.
While your FFR system may be different, most will give you a readout similar to what can be seen in Figure 1.
You can easily see a pressure before the lesion in red (Pa) and the pressure after the lesion in green (Pd). This results in an FFR of 0.71. These numbers are on the value of 1 (100%), which is assumed to be the normal flow through an artery with no obstruction.
So, how do we get these numbers? Let’s look at some generic steps in obtaining an FFR reading. Please keep in mind that your facility’s system may have a slightly different methodology. Your physicians may even have a different way of performing the procedure. In any case, always obtain instructions from the manufacturer of your FFR equipment.
Patient Preparation: It should be explained to the patient that a medication is being used to evaluate a small blockage that they may have. Explain that the medication is short acting, and sometimes makes people feel a little __________ (insert your own word to the effect of “yucky”, “odd”, “bad” or whatever you choose, based upon your conversation with the patient). Tell them that it is important that they let you know if they are feeling any changes during the test. Patients often complain of chest pain or tightness, and shortness of breath. The patient should be assured that these reactions are expected, and will dissipate quickly when the medication is stopped.
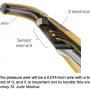 Equipment: Guiding catheters with side holes confound calculation and may underestimate maximum gradient1, because of pressures possibly being mixed with aortic pressures and not solely coronary artery pressure. The pressure wire will be a 0.014-inch wire with a transducer near the end of it, and it is important not to handle this end (Figure 2 shows the interior of the transducer).
Equipment: Guiding catheters with side holes confound calculation and may underestimate maximum gradient1, because of pressures possibly being mixed with aortic pressures and not solely coronary artery pressure. The pressure wire will be a 0.014-inch wire with a transducer near the end of it, and it is important not to handle this end (Figure 2 shows the interior of the transducer).
The wire should be packaged in some kind of “hoop” like other coronary wires.  In many cases, the analyzer will be connected to the wire BEFORE the wire is taken out of the hoop (Figure 3). This is done so that the machine can calibrate to the wire.
In many cases, the analyzer will be connected to the wire BEFORE the wire is taken out of the hoop (Figure 3). This is done so that the machine can calibrate to the wire.
A guiding catheter is inserted, seated, and the vessel viewed angiographically. Once the physician is comfortable with placement, the pressure wire should be  advanced to a point where the transducer of the wire is just outside the tip of the guiding catheter (Figure 4). At this point, most analyzers require an “equalization” process, to make sure that the pressure wire waveform is identical in pressure to the pressure you are showing from the guiding catheter (i.e., ‘AO’ pressure). If not equal, some troubleshooting will need to occur until the AO pressure and wire pressure are identical. Refer to your manufacturer’s “directions for use” resources.
advanced to a point where the transducer of the wire is just outside the tip of the guiding catheter (Figure 4). At this point, most analyzers require an “equalization” process, to make sure that the pressure wire waveform is identical in pressure to the pressure you are showing from the guiding catheter (i.e., ‘AO’ pressure). If not equal, some troubleshooting will need to occur until the AO pressure and wire pressure are identical. Refer to your manufacturer’s “directions for use” resources.
Once ‘equal’, the wire is advanced to a position where the transducer is past the lesion being investigated. Sometimes a gradient will already show. This is not considered a “positive” test until maximum hyperemia is achieved. Medication administration may begin at this point.
Medication: Adenosine is typically the medication of choice. It is administered at a rate of 140 mcg/kg/min. Because of a sustained hyperemia, some interventionalists prefer intravenous (IV) to intracoronary (IC) adenosine.2 Should the physician choose to administer IC adenosine, the recommended dose is 30 mcgs for the right coronary artery (RCA) and 40-60 mcgs for the left coronary artery (LCA).
The patient’s response to adenosine administered by IV infusion is generally attained within one minute, with maximum effects in two minutes. IC administration yields effects generally within 10 seconds3, and the effects of adenosine wear off quite quickly. Most effects will wear off in a few minutes. It’s our experience that continuous infusion seems to achieve more reliable results, as there is more information to process than with the short “hit-and-run” of IC injections. Remember that adenosine has a half-life of less than 20 seconds. That being said, both techniques are often employed; it varies by facility and physician.
Readers may already be aware that adenosine is often used to ‘break’ supraventricular tachycardia. Obviously, if the patient develops bradycardia after administration of adenosine, it should be assumed that maximal dose has been achieved and the physician should consider stopping the administration. Generally, treatment of the bradycardia is not needed, as the effects will wear off very quickly because of the short half-life of the medication (assuming the bradycardia is from the medication and not other reasons — always completely assess your patient to look for vasovagal responses).
 Theophylline and caffeine (or other xanthine derivatives) can act as antagonists to adenosine, which will prevent the patient from reaching maximum hyperemia. This should be discovered during the history and physical assessment the patient should receive before the procedure.
Theophylline and caffeine (or other xanthine derivatives) can act as antagonists to adenosine, which will prevent the patient from reaching maximum hyperemia. This should be discovered during the history and physical assessment the patient should receive before the procedure.
The value of FFR measurement can also be seen in a case at our facility. The patient presented with continuing chest pain on exertion. The RCA was found to  be totally occluded (probably chronic) (Figure 5) and a large posterior descending artery (PDA)/postero-lateral artery (PLA) system is noticed on angiography of the left arteries. The collaterals to the PDA/PLA appear to come from the left anterior descending coronary artery (LAD) and circumflex (Figure 6). The chronic total occlusion (CTO) appears moderate in length once the collateral and main vessels are visualized, with a calcific channel outlined.
be totally occluded (probably chronic) (Figure 5) and a large posterior descending artery (PDA)/postero-lateral artery (PLA) system is noticed on angiography of the left arteries. The collaterals to the PDA/PLA appear to come from the left anterior descending coronary artery (LAD) and circumflex (Figure 6). The chronic total occlusion (CTO) appears moderate in length once the collateral and main vessels are visualized, with a calcific channel outlined.
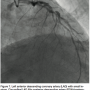 The LAD shows “lumpidy-bumps,” but nothing more than a 70-80% (remember, it is subjective) lesion (Figure 7). There are smaller lesions throughout the artery. The circumflex has a lesion that could be repaired with angioplasty (Figure 8).
The LAD shows “lumpidy-bumps,” but nothing more than a 70-80% (remember, it is subjective) lesion (Figure 7). There are smaller lesions throughout the artery. The circumflex has a lesion that could be repaired with angioplasty (Figure 8).
The discussion, in this case, was whether to fix the circumflex and bring the patient 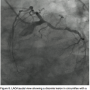 back later to attempt to open the CTO in the RCA. Further review of the LAD angiograms elicited suspicion regarding the present lesions. If the lesions were not significant, the angioplasty would proceed; however, if the lesions were significant, the patient would be sent to surgery for at least a three-artery bypass.
back later to attempt to open the CTO in the RCA. Further review of the LAD angiograms elicited suspicion regarding the present lesions. If the lesions were not significant, the angioplasty would proceed; however, if the lesions were significant, the patient would be sent to surgery for at least a three-artery bypass.
Since IVUS would clearly show a calcium burden, which was somewhat visible on the angiograms, we used a pressure wire to determine the physiological status of the artery. We also felt it was possible that the IVUS catheter may not completely reach the lesions, or be able to pass through the tortuosity in the LAD.
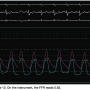
 Even upon placement of the wire, before adenosine administration, a gradient was present across the lesion (Figure 9) (Note: Herein, screen shots from the hemodynamic monitoring system will be used, because in our lab, we run the pressure wire [St. Jude Medical] through our hemodynamic system). As mentioned earlier, this doesn’t result in a “positive” test until maximum hyperemia is induced. To obtain an approximate gradient, we can divide the distal systolic to the proximal systolic to see the gradient (i.e., approximately 95 ÷ 110 = 0.86)
Even upon placement of the wire, before adenosine administration, a gradient was present across the lesion (Figure 9) (Note: Herein, screen shots from the hemodynamic monitoring system will be used, because in our lab, we run the pressure wire [St. Jude Medical] through our hemodynamic system). As mentioned earlier, this doesn’t result in a “positive” test until maximum hyperemia is induced. To obtain an approximate gradient, we can divide the distal systolic to the proximal systolic to see the gradient (i.e., approximately 95 ÷ 110 = 0.86)
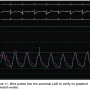
 After the adenosine administration, the gradient increased to a maximum of 0.63 (Figures 10-11). The decision was made to send the patient to surgery, since the LAD had physiologically significant disease as demonstrated by FFR.
After the adenosine administration, the gradient increased to a maximum of 0.63 (Figures 10-11). The decision was made to send the patient to surgery, since the LAD had physiologically significant disease as demonstrated by FFR.
FFR can also be used in multiple suspicious lesions in a vessel. The transducer would just be pulled back from one lesion to the other and additional measurements obtained, as outlined above.
 Again, your systems and processes to obtain these readings may be slightly different than what we describe. Always utilize the resources of your clinical representative from the manufacturer to help you with the proper use of the equipment if needed.
Again, your systems and processes to obtain these readings may be slightly different than what we describe. Always utilize the resources of your clinical representative from the manufacturer to help you with the proper use of the equipment if needed.
As Dr. Kern mentions in his “Editor’s Corner” this month, the combination of the FAME and FAME II trials will help provide direction for the treatment of patients with multi-vessel disease. FFR will also be a specific tool to document and validate appropriate stent usage. We would be remiss in not also mentioning that FFR could play an even more valuable role in the determination and validation of revascularization in ‘intermediate’ lesions. The recently published 2012 ACCF/SCAI/STS/AATS/AHA/ASNC/HFSA/SCCT (whew!) Appropriate Use Criteria for Coronary Revascularization Focused Update (https://content.onlinejacc.org/cgi/content/full/j.jacc.2011.12.001) outlines suggestions on when and when not to perform revascularization. This methodology is complex, and FFR is specifically mentioned, particularly for cases of one- or two-vessel coronary artery disease with borderline stenosis of 50% to 60%.4 One thing is certain: we will ALL hear more about FFR in the future.
We need your questions!
No question too big or small.
Please do not hesitate to send a
question to Cath Lab Digest via
cathlabdigest@aol.com or to me
directly at tginapp@rcisreview.com.
LETTER TO THE EDITOR
Re: January 2012’s “High-Paying Career Opportunities for Cath Lab Professionals”
Dear Cath Lab Digest,
I believe that the article about cath lab pay is inaccurate and misleading.
The survey polled directors, managers and supervisors, and put that pay in with staff members. This made the pay look extremely high and I believe that many cath lab workers will feel as a result of this article that they do not make enough. I am a manager of a cath lab and after reading this article, they feel that way. I would like to see this survey with the director, manager and supervisor pay taken out so that we can get a real picture of what cath lab staff makes nationally. Thank you!
Tom Maloney, MHA, RCIS, CLD editorial board member who edited the survey, responds:
I would agree with the editorial comment that management to include educator, director, manager, supervisor, and team leader should not be blended into the overall staff salary. This would be due to the fact that these positions of leadership should be compensated at a higher rate and could falsely elevate a staff hourly wage, causing a management crisis where employees feel that they are underpaid. Realizing that would be a limitation, this survey does not blend management with staff salary.
This survey does provide a blend of 72% (n=1,112) staff members and 28% (n=434) management. However, the survey results are broken out to reflect staff only and management only. For example, an RCIS staff mem- ber, on average, for all regions, earns $29.75, which is reflected in the chart “Hourly Wages by Credentials and Regions” and matches the same figure for “Staff Employee: Average Hourly Wage by Certification”. If, however, this same RCIS was to assume a management position, the rate would increase to $32.87, which reflects that a promotion into leadership is duly compensated for. What is not possible is to provide deeper cuts into wag- es for management like those for staff, for years of service and by different titles, due to the smaller sample size of management.
Kind regards,
Tom Maloney, MHA, RCIS
References
- Libby P, Bonow RO, Mann DL, Zipes DP. Braunwald’s Heart Disease: A Textbook of Cardiovascular Medicine. 8th Edition. Philadelphia, Pennsylvania: Elsevier Science; 2007.
- Baim DS, Grossman W (eds). Grossman’s Cardiac Catheterization, Angiography, and Intervention, 6th edition. Philadelphia: Lippincott Williams and Wilkins; 2000.
- De Bruyne B, Pijls NH, Barbato E, Bartunek J, et al. Intracoronary and intravenous adenosine 5’-triphosphate, adenosine, papaverine, and contrast medium to assess fractional flow reserve in humans. Circulation 2003 Apr 15;107(14):1877-1883.
- American College of Cardiology Foundation Appropriate Use Criteria Task Force; Society for Cardiovascular Angiography and Interventions; Society of Thoracic Surgeons; American Association for Thoracic Surgery; American Heart Association; American Society of Nuclear Cardiology; Society of Cardiovascular Computed Tomography; American Society of Echocardiography; Heart Rhythm Society, Patel MR, Dehmer GJ, Hirshfeld JW, Smith PK, Spertus JA. ACCF/SCAI/STS/AATS/AHA/ASNC/HFSA/SCCT 2012 Appropriate Use Criteria for Coronary Revascularization Focused Update. J Am Coll Cardiol 2012 Jan 23. [Epub ahead of print].
- Briguori C, Anzuini A, Airoldi F, Gimelli G, et al. Intravascular ultrasound criteria for the assessment of the functional significance of intermediate coronary artery stenoses and comparison with fractional flow reserve. Am J Cardiol 2001;87(2):136-141.
- Jasti V, Ivan E, Yalamanchili V, Wongpraparut N, Leesar MA. Correlations between fractional flow reserve and intravascular ultrasound in patients with ambiguous left main coronary artery stenosis. Circulation 2004 Nov 2;110(18):2831-2836.










