ADVERTISEMENT
Accurate Hemodynamic Data Recording: Recognizing and Overcoming Hemodynamic “Glitches”
 From Wikipedia: “A glitch is a short-lived fault in a system. It is often used to describe a transient fault that corrects itself, and is therefore difficult to troubleshoot. The term is particularly common in the computing and electronics industries…although it is applied to all types of systems, including human organizations and nature. The term derives from the German glitschig, meaning ‘slippery’, possibly entering English through the Yiddish term ‘glitsh’.” How appropriate for hemodynamic recording problems.
From Wikipedia: “A glitch is a short-lived fault in a system. It is often used to describe a transient fault that corrects itself, and is therefore difficult to troubleshoot. The term is particularly common in the computing and electronics industries…although it is applied to all types of systems, including human organizations and nature. The term derives from the German glitschig, meaning ‘slippery’, possibly entering English through the Yiddish term ‘glitsh’.” How appropriate for hemodynamic recording problems.
It goes without saying that I greatly enjoy teaching our fellows and staff about cardiac hemodynamics acquired during cath lab procedures, both for normal and diseased states. However, I recognize the difficulties (“glitches”) the cath lab nurse/tech faces when the hemodynamic recording system is not “cooperating” (i.e. too many clicks of the mouse to get to the right button to collect the properly labeled pressure wave) or the inputs to the system are not cooperating (i.e. not correctly plugged in, loosely connected, short-circuited wires or broken connector cables, etc.) or finally, that the operators at the table are not cooperating (i.e. the wrong pressure is connected, the lines are not flushed, there are bubbles, or the operators are moving quicker than the technologist can follow.) It is with these problems in mind that I want to discuss the glitches that prevent getting great hemodynamic tracings for accurate clinical decisions. While one of the oldest problems of the cath lab, hemodynamic recording remains an issue of continuous quality improvement even with the modernization and automation of hemodynamic recorders.
Where do “glitches” come from?
 Acquiring accurate hemodynamics depends on the level of interest of both the operators and recording technologists. Inaccurate hemodynamics involve failing to recognize hemodynamic “glitches”, those stuttering steps which produce artifact or errors. “Glitches” commonly come from two places (Figure 1). The first is the initial technical set up in the lab before the patient arrives, beginning at the pressure transducer back through the connection tubing, all the way to the recorder. The second source comes from the interaction (read: cooperation) among personnel doing the recording, and the operator and team members assisting at the table in the acquisition of the pressure signals. I’ll review some of these issues and share a few hemodynamic tracings which might help the team recognize some uncommon glitches.
Acquiring accurate hemodynamics depends on the level of interest of both the operators and recording technologists. Inaccurate hemodynamics involve failing to recognize hemodynamic “glitches”, those stuttering steps which produce artifact or errors. “Glitches” commonly come from two places (Figure 1). The first is the initial technical set up in the lab before the patient arrives, beginning at the pressure transducer back through the connection tubing, all the way to the recorder. The second source comes from the interaction (read: cooperation) among personnel doing the recording, and the operator and team members assisting at the table in the acquisition of the pressure signals. I’ll review some of these issues and share a few hemodynamic tracings which might help the team recognize some uncommon glitches.
The initial setup
The initial setup should be simplified whenever possible. The team should color or number code cables to matching inputs and output sockets. Make sure that the appropriate cables are connected to the right transducers and that the cables are correctly labeled, so that the transducers can be easily identified by the operators (especially when two or more transducers will be used). Ensure that the transducer zero heights are set to the mid-chest of the patient, at least at the beginning of the procedure. External manual manometer transducer calibrations are generally unnecessary as in the past. However, transducer connections with leaking fluid, bubbles, and excessively floppy tubing will still produce errors.
At the table, the operators should know which transducers correspond to the right and left heart catheters, and should share this information with the technologist. The recording technologist must make sure those signals are correctly labeled in the recording system. Zero calibrations must be accepted by the hemodynamic recording system before accurate recordings can be made.
Several years ago, we acquired a new hemodynamic recording system (the Philips Sensis). Our frustration level rose quickly, because the recording technologist must move quickly through multiple clicks of the mouse to obtain a simple signal and zero. Not only is this is a source of frustration, but is often source of error and large contributor to the glitches in the smooth acquisition of the hemodynamic signals and procedural flow.
Signal acquisition during the procedure
The second major source of “glitches” is the inability of recording technologist to follow the physician as he or she moves through the different steps of the procedure requiring recording pressures in different locations (at different pressure scales and sweep speeds) within the heart. Clear communication in the lab will often solve this problem. For example, the simplest and most commonly acquired hemodynamic signal is that of left ventricular pressure before and after ventriculography. By custom, both systolic and end-diastolic pressures are recorded at each point. Accurate acquisition of the signal, of course, requires the operator to have the catheter in the right place in the ventricle and be free of frequent premature ventricular contractions that may produce artifact in the signal. The catheter must be flushed and connected tightly, free of bubbles and accurately zeroed. Once these steps are taken by the operator, the recording technologist can communicate to the operator when she or he is ready to record. At this point, control is turned over to the technologist for data collection. It is essential to have a smooth running process by which the hemodynamics will be accurately recorded and stored.
Hemodynamic conundrums
 Let’s look at some hemodynamic tracings to understand why the interpretation of the data may produce erroneous conclusions. Figure 2 shows two examples of left ventricular pressure. The ideal left ventricular and aortic pressures (left side, Figure 2) have been acquired through high fidelity micromanometer transducers. Beginning at the QRS on the ECG, the tracing shows a clear end-diastolic ‘a’ wave followed by the left ventricular (LV) upstroke meeting the aortic pressure upstroke. Continuing after the aortic valve opens, the anacrotic shoulder shows a small (2mmHg) impulse gradient (red arrow) between the aortic and left ventricular pressure, a normal finding. As ejection finishes, relaxation (at the T wave) causes both pressures to fall until the aortic valve closes, producing the dichrotic notch. After which, the left ventricular pressure continues to fall rapidly to the lowest point in the cardiac cycle. The LV pressure gradually increases over the diastolic period until the electrical P-wave produces a mechanical ‘a’ wave, ending at the left ventricular end-diastolic pressure (LVEDP).
Let’s look at some hemodynamic tracings to understand why the interpretation of the data may produce erroneous conclusions. Figure 2 shows two examples of left ventricular pressure. The ideal left ventricular and aortic pressures (left side, Figure 2) have been acquired through high fidelity micromanometer transducers. Beginning at the QRS on the ECG, the tracing shows a clear end-diastolic ‘a’ wave followed by the left ventricular (LV) upstroke meeting the aortic pressure upstroke. Continuing after the aortic valve opens, the anacrotic shoulder shows a small (2mmHg) impulse gradient (red arrow) between the aortic and left ventricular pressure, a normal finding. As ejection finishes, relaxation (at the T wave) causes both pressures to fall until the aortic valve closes, producing the dichrotic notch. After which, the left ventricular pressure continues to fall rapidly to the lowest point in the cardiac cycle. The LV pressure gradually increases over the diastolic period until the electrical P-wave produces a mechanical ‘a’ wave, ending at the left ventricular end-diastolic pressure (LVEDP).
Unfortunately, we rarely see this pressure tracing with our fluid-filled systems, as shown on the right side of Figure 2. In this tracing, the systemic pressure was obtained from the side arm of the femoral sheath. The LV pressure is measured with the fluid-filled pigtail catheter. The timing delay of femoral artery upstroke after LV pressure signifies location (distant from the central aortic position) and quality (i.e., compliance) of the vasculature between sheath and central aorta. Also note that aortic pressure is higher than the LV systolic pressure (called overshoot or pressure amplification), again a normal finding, without which one must question whether there is some peripheral vascular disease, sheath kink, or another reason why the femoral artery pressure cannot exceed that of LV pressure.
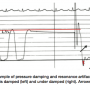 Also, note the difference in the fidelity (smoothness) of the pressure wave signal between the high fidelity tracings, the femoral artery and fluid LV pressures. The sharp, short resonant artifacts (yellow arrow) seen on the fluid-filled LV pressure tracing occur as the catheter moves in the ventricle, indicating an under-damped system. Another example of an under-damped pressure is shown in Figure 3. After the left ventricular pressure is flushed (left), its smooth configuration changes and shows the exaggerated pressure “fling” (resonant artifacts) due to a bubble in the fluid-filled transducer line (right). This catheter should be again flushed, connections tightened, and properly damped to use the signal correctly. You can appreciate the fact that it might be difficult to get an accurate peak systolic pressure from this tracing, not knowing exactly where systole is. Likewise, for diastole, there is no such thing as a negative diastolic pressure of the magnitude shown here. A properly flushed system should eliminate under damping.
Also, note the difference in the fidelity (smoothness) of the pressure wave signal between the high fidelity tracings, the femoral artery and fluid LV pressures. The sharp, short resonant artifacts (yellow arrow) seen on the fluid-filled LV pressure tracing occur as the catheter moves in the ventricle, indicating an under-damped system. Another example of an under-damped pressure is shown in Figure 3. After the left ventricular pressure is flushed (left), its smooth configuration changes and shows the exaggerated pressure “fling” (resonant artifacts) due to a bubble in the fluid-filled transducer line (right). This catheter should be again flushed, connections tightened, and properly damped to use the signal correctly. You can appreciate the fact that it might be difficult to get an accurate peak systolic pressure from this tracing, not knowing exactly where systole is. Likewise, for diastole, there is no such thing as a negative diastolic pressure of the magnitude shown here. A properly flushed system should eliminate under damping.
Aortic valve gradient measurement
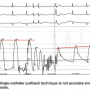 An accurate LV-aortic gradient should be measured with two transducers. Figure 4 shows the effect of single catheter pullback to assess the aortic valve gradient. As has been discussed in these pages before, best accuracy for aortic stenosis (AS) requires simultaneous measurement of aortic and left ventricular pressure. It is difficult if not impossible to tell what the pressure gradient may be on a single-catheter pullback recording. For comparison to the above under-damped tracing, the fidelity of this left ventricular pressure tracing is good, as noted by the low-frequency vibrations of diastole without the overshoot of systolic pressure fling.
An accurate LV-aortic gradient should be measured with two transducers. Figure 4 shows the effect of single catheter pullback to assess the aortic valve gradient. As has been discussed in these pages before, best accuracy for aortic stenosis (AS) requires simultaneous measurement of aortic and left ventricular pressure. It is difficult if not impossible to tell what the pressure gradient may be on a single-catheter pullback recording. For comparison to the above under-damped tracing, the fidelity of this left ventricular pressure tracing is good, as noted by the low-frequency vibrations of diastole without the overshoot of systolic pressure fling.
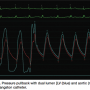 An easy catheter for measuring LV-aortic gradients is the dual-lumen Langston catheter (Vascular Solutions), with which simultaneous aortic and left ventricular pressures can be easily acquired (Figure 5). However, since the aortic lumen of this catheter is so small, the pressures may not be identical at all times. Flushing and correct zeroing are important to get the best signal quality from this catheter. Figure 5 shows that on pullback across the aortic valve, there is a slight signal drift of the small aortic pressure lumen that does not match the larger aortic pressure lumen either in diastole or systole. This particular recording would then require flushing and perhaps re-calibration for accuracy.
An easy catheter for measuring LV-aortic gradients is the dual-lumen Langston catheter (Vascular Solutions), with which simultaneous aortic and left ventricular pressures can be easily acquired (Figure 5). However, since the aortic lumen of this catheter is so small, the pressures may not be identical at all times. Flushing and correct zeroing are important to get the best signal quality from this catheter. Figure 5 shows that on pullback across the aortic valve, there is a slight signal drift of the small aortic pressure lumen that does not match the larger aortic pressure lumen either in diastole or systole. This particular recording would then require flushing and perhaps re-calibration for accuracy.
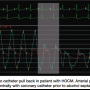 Patients with hypertrophic obstructive cardiomyopathy (HOCM) require measurement of the aortic and LV intracavitary gradient. However, the recording of intracavitary gradient may not be accurate using a pigtail catheter, since multiple side holes on the catheter shaft may be moved across the area of the gradient, with a potential for falsely low distal LV pressure. To avoid this problem, we use a Halo catheter (Vascular Dynamics) that has all the pressure holes around the perpendicular ring. Figure 6 shows that on slow pullback of the Halo catheter from the distal cavity back just under the valve, the intracavitary gradient disappears. Under the aortic valve, the systolic aortic pressure matches the left ventricular pressure. This nice example of a HOCM tracing also shows the spike and dome pattern, and near-vertical upstroke (as opposed to delayed upstroke of AS, see Figure 5) on the aortic pressure characteristic of HOCM hemodynamics.
Patients with hypertrophic obstructive cardiomyopathy (HOCM) require measurement of the aortic and LV intracavitary gradient. However, the recording of intracavitary gradient may not be accurate using a pigtail catheter, since multiple side holes on the catheter shaft may be moved across the area of the gradient, with a potential for falsely low distal LV pressure. To avoid this problem, we use a Halo catheter (Vascular Dynamics) that has all the pressure holes around the perpendicular ring. Figure 6 shows that on slow pullback of the Halo catheter from the distal cavity back just under the valve, the intracavitary gradient disappears. Under the aortic valve, the systolic aortic pressure matches the left ventricular pressure. This nice example of a HOCM tracing also shows the spike and dome pattern, and near-vertical upstroke (as opposed to delayed upstroke of AS, see Figure 5) on the aortic pressure characteristic of HOCM hemodynamics.
Computer shifting of signals
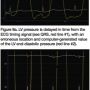
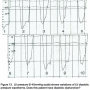
 New hemodynamic recording systems may be set to shift individual tracings to 40-120 msec in time. This action is most commonly used with the femoral pressure shifted to match LV pressure for computation of AS gradients. Figure 7 shows an example of the computer-shifted tracing of femoral pressure, shifted back to the left with an upstroke. In fact, it was over-shifted with the femoral pressure preceding that of LV pressure, a physical impossibility. The recording technologist should recognize the fact that this will produce an artificially low gradient and reset the computer for unshifted calculations of aortic valve area.
New hemodynamic recording systems may be set to shift individual tracings to 40-120 msec in time. This action is most commonly used with the femoral pressure shifted to match LV pressure for computation of AS gradients. Figure 7 shows an example of the computer-shifted tracing of femoral pressure, shifted back to the left with an upstroke. In fact, it was over-shifted with the femoral pressure preceding that of LV pressure, a physical impossibility. The recording technologist should recognize the fact that this will produce an artificially low gradient and reset the computer for unshifted calculations of aortic valve area.
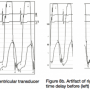 Similarly, when using multiple transducers, the timing of each pressure amplifier signal display can be individualized and may be different. There is a default setting within the recording system to set both transducer clocks to zero, such that the timing of signals would then be displayed at the same time (i.e., without delay of one or the other signal). Figure 8 shows the effect of a pressure signal time delay for the right and left heart transducers. You can see that although the LV pressure signal is correctly timed to the QRS with the corresponding LVEDP at the correct location, the right heart pressure is shifted more than 100 msec to the right (Figure 8b). When corrected, this signal then matches the appropriate left and right ventricular pressure synchrony.
Similarly, when using multiple transducers, the timing of each pressure amplifier signal display can be individualized and may be different. There is a default setting within the recording system to set both transducer clocks to zero, such that the timing of signals would then be displayed at the same time (i.e., without delay of one or the other signal). Figure 8 shows the effect of a pressure signal time delay for the right and left heart transducers. You can see that although the LV pressure signal is correctly timed to the QRS with the corresponding LVEDP at the correct location, the right heart pressure is shifted more than 100 msec to the right (Figure 8b). When corrected, this signal then matches the appropriate left and right ventricular pressure synchrony.
 A similar effect can be seen In Figure 9a, where the LV pressure is delayed in time from the ECG timing signal (see QRS, red line #1), with an erroneous location and computer-generated value of the LV end-diastolic pressure (red line #2). The recording system technologist should observe the ECG and corresponding pressures to identify this phenomenon and reset the pressure amplifier timing default settings back almost to zero (Figure 9b).
A similar effect can be seen In Figure 9a, where the LV pressure is delayed in time from the ECG timing signal (see QRS, red line #1), with an erroneous location and computer-generated value of the LV end-diastolic pressure (red line #2). The recording system technologist should observe the ECG and corresponding pressures to identify this phenomenon and reset the pressure amplifier timing default settings back almost to zero (Figure 9b).
Heart rate changes
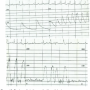 The recording technologist should always be alert for changes in heart rate. The bradycardia shown in Figure 10 (top panel) is the result of a significant new problem in the cath lab. The bradycardia is due to complete heart block, with the P-waves on the electrocardiogram moving through the tracing. When sinus rhythm is restored (Figure 10, right side), the blood pressure rises dramatically. The accompanying LV pressure (bottom panel, Figure 10) shows failure of the pacemaker to capture (beat # 4 from the left) and then LV activity again
The recording technologist should always be alert for changes in heart rate. The bradycardia shown in Figure 10 (top panel) is the result of a significant new problem in the cath lab. The bradycardia is due to complete heart block, with the P-waves on the electrocardiogram moving through the tracing. When sinus rhythm is restored (Figure 10, right side), the blood pressure rises dramatically. The accompanying LV pressure (bottom panel, Figure 10) shows failure of the pacemaker to capture (beat # 4 from the left) and then LV activity again 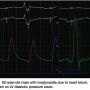 resumes for transient evident LV contractions, and then, finally, loss of the QRS on the right side of the tracing demonstrates asystole. This patient ultimately needed a permanent pacemaker. Everybody in the lab should be observing the hemodynamic monitors for these kinds of problems. Figure 11 shows the effect of heart block on the LV pressure tracing. The ‘a’ waves can be seen marching through the LV pressure.
resumes for transient evident LV contractions, and then, finally, loss of the QRS on the right side of the tracing demonstrates asystole. This patient ultimately needed a permanent pacemaker. Everybody in the lab should be observing the hemodynamic monitors for these kinds of problems. Figure 11 shows the effect of heart block on the LV pressure tracing. The ‘a’ waves can be seen marching through the LV pressure.
Check diastolic dysfunction
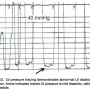 Finally, the configuration of the LV diastolic pressure is useful to assess diastolic dysfunction (Figure 12). The diastolic LV waveform can be confused with pigtail catheter movement across the aortic valve. The lowest pressure occurs late in mid-diastole, where pressure is falling while the ventricle is filling. In Figure 13, the LV diastolic pressure shows a characteristic pattern of diastolic dysfunction, with impaired relation occurring over the respiratory cycle (beats 3-7), and then returning to normal LV configuration. The tracing shows what happens when one of the catheter side holes has moved out of the LV across the aortic valve with respiration. The operators could easily detect this by the change in catheter position during the set up for ventriculography.
Finally, the configuration of the LV diastolic pressure is useful to assess diastolic dysfunction (Figure 12). The diastolic LV waveform can be confused with pigtail catheter movement across the aortic valve. The lowest pressure occurs late in mid-diastole, where pressure is falling while the ventricle is filling. In Figure 13, the LV diastolic pressure shows a characteristic pattern of diastolic dysfunction, with impaired relation occurring over the respiratory cycle (beats 3-7), and then returning to normal LV configuration. The tracing shows what happens when one of the catheter side holes has moved out of the LV across the aortic valve with respiration. The operators could easily detect this by the change in catheter position during the set up for ventriculography.
 Recognition and avoidance of hemodynamic glitches should improve your confidence in the diagnosis made by hemodynamic data in your lab. I hope this discussion and these tracings have illuminated some of the problems that can be remedied by close attention to the hemodynamic data acquired in the cath lab.
Recognition and avoidance of hemodynamic glitches should improve your confidence in the diagnosis made by hemodynamic data in your lab. I hope this discussion and these tracings have illuminated some of the problems that can be remedied by close attention to the hemodynamic data acquired in the cath lab.
Disclosure: Dr. Kern reports that he is a speaker for Volcano Therapeutics and St. Jude Medical, and is a consultant for Merit Medical and InfraReDx, Inc.










