3D Rendering in Transcatheter Aortic Valve Replacement
What has been your experience with transcatheter aortic valve replacement (TAVR)?
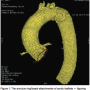 I have been fortunate enough to work on TAVR since the beginning. I was a fellow under Alain Cribier, the creator of this technique. We began our program at Nantes University Hospital about five years ago, and have been working with different generations of the various devices, beginning with a large device with a 24 French (F) sheath to a 16 F sheath today. We have implanted at least 150 patients in our cath lab, and are very happy with this new technique; it saves lives. TAVR has shown a dramatic reduction in mortality as compared to medical treatment for high-risk surgical patients. It has been validated in the New England Journal of Medicine for high-risk aortic stenosis patients unable to undergo surgery.1
I have been fortunate enough to work on TAVR since the beginning. I was a fellow under Alain Cribier, the creator of this technique. We began our program at Nantes University Hospital about five years ago, and have been working with different generations of the various devices, beginning with a large device with a 24 French (F) sheath to a 16 F sheath today. We have implanted at least 150 patients in our cath lab, and are very happy with this new technique; it saves lives. TAVR has shown a dramatic reduction in mortality as compared to medical treatment for high-risk surgical patients. It has been validated in the New England Journal of Medicine for high-risk aortic stenosis patients unable to undergo surgery.1
Most of our TAVR procedures are performed through the transfemoral approach and these procedures are done in a regular cath lab. For the transapical approach, we work in a surgical room, but we soon hope to be able to work in hybrid operating rooms, which can enable us to convert the patient into a surgical approach if necessary.
Does your facility use both the self-expandable CoreValve (Medtronic) and the balloon-expandable Sapien valve (Edwards)?
Yes. We have been undergoing the learning curves for both in our institution and we are pleased to have the option to implant a self-expandable valve or a balloon-expandable valve as needed. These two choices enable us to treat a larger variety of patients, because one valve cannot treat the full range of annulus sizing we see in our patients. Some indications are shared by both valves, but there are also extreme indications for one type or the other, especially for a large-sized annulus, above 25 mm, which is best treated with a self-expandable valve.
You now use 3D imaging with the GE Innova HeartVision for your TAVR procedures. Where does 3D imaging prove most useful?
Precise patient selection is important. There are concerns regarding vascular access. When you have decided to treat these patients, who are high-risk patients for surgery, correct valve sizing must be determined. Also, it is important to determine the optimal angulation for valve deployment and valve delivery.
Can you share more about your use of 3D imaging for vascular access?
3D imaging for vascular access helps us to pinpoint the location of the common femoral artery, used as a puncture site, and also the relationships between the artery and the puncture site regarding the pelvis bones. We can further define any tortuousity, angulation, and calcification at the site as well, all aspects that are very important to take into account when aortic stenosis patients undergo access, because mortality is related to vascular complications. 3D imaging helps us greatly in these assessments.
Do you find that difficult anatomy is the norm with these types of patients?
Yes, most of these patients are quite old, above 80, sometimes 85, or nonagenarians. They have highly calcified, tortuous, tight and difficult access. To have a successful procedure, we must prevent a vascular complication. The first step is knowing exactly how to access these patients, whether percutaneously or with a surgical approach.
How is the 3D imaging incorporated into the procedure and patient assessment?
In our institution, the 3D imaging is a reconstruction based on a computed tomography (CT) scan. One may also do 3D angiography with Innova 3D. By visualizing the anatomy in 3D, we cannot only plan out our procedure, but also locate the coronary ostium, and assess the aortic valve plane and the iliac artery lumen diameter. We also use 3D imaging to guide the procedure, because once the patient’s anatomy from the groin to the aortic valve is assessed with 3D imaging, a 3D reconstruction can be fused to regular fluoroscopic imaging. This fused image will follow any table panning or gantry angulations that are changed throughout the procedure. To minimize the difference between the fluoro image and the 3D model, we may also utilize the ECG-gated and Image Stabilization functions. We find it a great help for valve analysis and assessment, device plane assessment, and valve delivery.
Have you found any reduction in contrast media volumes?
Certainly, this type of imaging is helpful with contrast dye reduction at the implantation time and we consider it is crucial, since our high-risk patients usually suffer from renal insufficiency. We are typically using 40-60 mL of contrast medium for such a complex procedure, which is quite low.
Can you share more about the impact of 3D imaging on workflow?
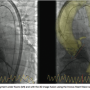 The beginning is the assessment of vascular access for the patient, and our 3D imaging leaves us with very few surprises, because before placing the catheters and valve into the femoral arteries of the patient, we know exactly where we are going to find difficulties — where it is going to be tortuous and/or calcified. Second, what is important in terms of reducing procedural time is that we directly find, via the 3D reconstruction, the valve plane and the correct angulation for valve deployment, and the correct angulation of the gantry. Angulation for valve deployment is predetermined by 3D imaging, so now we don’t lose any time using fluoroscopy to find the correct valve plane assessment for valve delivery. This aspect of the 3D pre-procedure assessment is a very important time reduction. A retrospective analysis has demonstrated that there is a wide variety of anatomy requiring specific choice of angulations.
The beginning is the assessment of vascular access for the patient, and our 3D imaging leaves us with very few surprises, because before placing the catheters and valve into the femoral arteries of the patient, we know exactly where we are going to find difficulties — where it is going to be tortuous and/or calcified. Second, what is important in terms of reducing procedural time is that we directly find, via the 3D reconstruction, the valve plane and the correct angulation for valve deployment, and the correct angulation of the gantry. Angulation for valve deployment is predetermined by 3D imaging, so now we don’t lose any time using fluoroscopy to find the correct valve plane assessment for valve delivery. This aspect of the 3D pre-procedure assessment is a very important time reduction. A retrospective analysis has demonstrated that there is a wide variety of anatomy requiring specific choice of angulations.
We first began doing TAVR without 3D imaging and had some good results. But now we find that using 3D imaging helps us in reducing contrast medium and procedural time, and finally, helps us improve our successful valve placement and delivery.
We certainly have to further validate this technique and software on larger cohorts of patients, and find whether there is a significant reduction in the parameters I mentioned, i.e., reduction in contrast medium, and finally, good results in terms of paravalvular leakage, and so on. We also have to improve the imaging in terms of precision.
Three-dimensional imaging in TAVR will be very important for experienced interventional cardiologists, but also for people who are entering the learning curve for this procedure. Physicians will feel themselves more confident if they have software that helps to accurately place and deploy valves in the right position. The integration of the 3D image in the procedure workflow was seamless. A single GE workstation provides very comprehensive tools for the case preparation and once ready, the image fusion is initiated by a simple click with display in the exam room. Any specific tuning, such as image contrast between the fluoro and 3D image in the fusion, can be performed from tableside.
Any final thoughts?
We have to imagine the heart not only in two dimensions, but three dimensions, because the annulus valve is a complex, 3D anatomic structure. Thinking only in terms of two dimensions is losing the final dimension that helps us to find the correct sizing and prosthesis. Based on our own experience, we have found 3D imaging helps the operator obtain better sizing for valves and leads to overall better outcomes for patients. This software is dedicated to providing for a better assessment, but also to help the physician starting the TAVR learning curve. It is important to select the correct patient, assess vascular access precisely, and finally, to deliver the valve properly, and this kind of software helps the physician to do so.
Dr. Tirouvanziam can be contacted at ashok.tirouvanziam@chu-nantes.fr.
Reference
- Leon MB, Smith CR, Mack M, Miller DC, et al; PARTNER Trial Investigators. Transcatheter aortic-valve implantation for aortic stenosis in patients who cannot undergo surgery. N Engl J Med 2010 Oct 21;363(17):1597-1607.
For Further Reading
- Holmes DR Jr, Mack MJ; Writing Committee. Transcatheter valve therapy: a professional society overview from the American College of Cardiology Foundation and the Society of Thoracic Surgeons. Ann Thorac Surg 2011 Jul;92(1):380-389.
- Eltchaninoff H, Prat A, Gilard M, Leguerrier A, et al; FRANCE Registry Investigators. Transcatheter aortic valve implantation: early results of the FRANCE (FRench Aortic National CoreValve and Edwards) registry. Eur Heart J 2011 Jan;32(2):191-197.










