A Primer on Artificial Intelligence (AI). Plus: Is Your Job on the Line?
 Artificial intelligence (AI) is no longer science fiction. AI is a discipline that has been under rapid development for the last three decades. As with most scientific innovations, AI has experienced tribulations ahead of its present burgeoning applications. However, with the current profusion in computer storage and data processing capacity, AI is on the verge of exponential growth with an infinite range of applications, including computer vision, robotics, natural language processing, and machine learning. In the last ten years, smart TVs, smartphones, smart houses, and smart cars have become part of our daily lives, and medicine is not the exception. AI is used to design evidence-based treatment plans for cancer patients, instantly analyze results from medical tests to refer to the appropriate specialist immediately, and conduct scientific research for drug discovery.1 AI has already been part of our lives for over two decades.
Artificial intelligence (AI) is no longer science fiction. AI is a discipline that has been under rapid development for the last three decades. As with most scientific innovations, AI has experienced tribulations ahead of its present burgeoning applications. However, with the current profusion in computer storage and data processing capacity, AI is on the verge of exponential growth with an infinite range of applications, including computer vision, robotics, natural language processing, and machine learning. In the last ten years, smart TVs, smartphones, smart houses, and smart cars have become part of our daily lives, and medicine is not the exception. AI is used to design evidence-based treatment plans for cancer patients, instantly analyze results from medical tests to refer to the appropriate specialist immediately, and conduct scientific research for drug discovery.1 AI has already been part of our lives for over two decades.
This article will serve as an introduction for cardiologists and for other healthcare providers regarding AI’s presence in the world of medicine. Without evading deeper technical and mathematical explanations, it will also endeavor to answer the most critical question — is your job on the line as a result of burgeoning AI in various areas of healthcare?
What is Artificial Intelligence (AI)?
AI is the science of giving computers the opportunity to acquire human reasoning skills that normally rely on superior brain functions. Its foundations include mathematics, logic, philosophy, probability, linguistics, neuroscience, and decision theory. Many fields fall under the umbrella of AI, such as computer vision, robotics, machine learning, and natural language processing.1
Machine Learning
 Machine learning is a subfield of AI where the goal is to enable computers to learn on their own. A machine learning algorithm is trained to identify patterns in observed data, build models that explain the phenomenon, and predict effects without having explicit pre-programmed rules and models.1
Machine learning is a subfield of AI where the goal is to enable computers to learn on their own. A machine learning algorithm is trained to identify patterns in observed data, build models that explain the phenomenon, and predict effects without having explicit pre-programmed rules and models.1
Machine learning has many interconnected subfields. Cardiologists are using machine learning in varied forms. For example, cardiologists and electrophysiologists are cooperating to develop accurate algorithms to assess electrocardiogram (ECG) patterns using machine learning models (MLM). Among the available MLMs, the following applications have been used for ECG functions: regression, fuzzy set theory (FST), support vector machine (SVM), random forest, rough set theory (RST), Markov model (MM), artificial neural networks (ANN), and genetic algorithms (GA), among others (Figure 1A).
A simple way to comprehend machine learning is by comparing it to the creation of a clinical trial:
1. Preclinical Phase
a. Determine the precise topic that needs research and develop an algorithm that is mandatory for all machine learning processes.
b. Select the data set that will be the input given to the machine learning algorithm and precisely identify and define the variables or features of these data in relation to the object of study.
c. Incorporate ideal methods, in terms of relevance. This is, perhaps, the most critical step of machine learning and it involves developing a mathematical model. In medicine, it would parallel the selection of the clinical protocol to apply. With this component in place, we are ready to train the machine learning algorithm.
2. Phase I
Test the MLM to process data. For this, the input must be acquired and prepared. In addition, the features that the algorithm should recognize must be precisely defined. Instead of testing a drug on patients, for example, test the MLM on data in order to train the model to recognize the specific features.
3. Phase II
Test the model using a random data set. How well does the MLM perform in recognizing the features? Some MLM learn new relationships among variables while completing the processing of the features.
Compare the new MLM with pre-existing ones that have the same goal. This step is usually the most controversial, since the data used for training usually varies for each model. The MLMs are evaluated according to their accuracy, specificity, and sensitivity, as shown in Table 1.
Data Extraction
In order to explore the system, engineers must find the way to translate an ECG into a language that a machine learning model can understand as input. This process takes three steps: pre-processing, feature extraction, and classification.
Pre-processing is the transformation of the data into a configuration that is simpler to process by the MLM. Feature extraction refers to the reduction in the size of properties that are needed to describe voluminous data, done by defining specific features that the MLM will process from the given input. Finally, this information must be labeled so the MLM can classify the input. As an example, the process assigns the label “4” to an image that contains a handwritten number 4.
 Figure 2 demonstrates a relatively simple example of ECG processing. The baseline drift in the rhythm must first be removed before feature extraction. Assuming that the task is to identify ECG waves, then the features to assess will be height, length, and shape. Based upon the features that are presented, the MLM will classify the input into P wave, QRS, and T wave.
Figure 2 demonstrates a relatively simple example of ECG processing. The baseline drift in the rhythm must first be removed before feature extraction. Assuming that the task is to identify ECG waves, then the features to assess will be height, length, and shape. Based upon the features that are presented, the MLM will classify the input into P wave, QRS, and T wave.
Now that we have described the process of data extraction, we will now review individual ML algorithm models:
Supervised Learning
In supervised learning tasks, we begin with a dataset containing training examples with associated “correct” labels. For example, when learning to classify handwritten digits, a supervised learning algorithm takes thousands of pictures of the written samples — digits along with labels containing the correct number that each image represents. The algorithm will then learn the relationship between the images and their associated numbers, and apply this knowledge to classify completely new images (without labels) that the machine has not seen before. This is the defined methodology used when depositing checks via mobile phone by first taking a picture!
Support Vector Machine
A Support Vector Machine (SVM) is a discriminative classifier strictly defined by a separating hyperplane. In other words, given labeled training data (supervised learning), the algorithm outputs an optimal hyperplane that categorizes new specimens. In 2-dimensional space, this hyperplane is a line dividing a plane in two parts, where each class is placed on either side.2 In essence, it typically solves the problem classifying the data into two classes (Figure 1B).1 Table 1 shows a report that used this approach to diagnose acute coronary syndrome.
Unsupervised Learning
The goal for unsupervised learning is to model the underlying structure or distribution in the data in order to learn more about the features and relationships of the data. Unsupervised learning means that the input data is not labeled; therefore, the output is not defined as it is in Supervised Learning. Rather, the output is constructed as the MLM learns the hidden structures within the data. The construct of Supervised Learning implies that it is difficult to design metrics that assess how well the MLM is performing; therefore, the performance of the MLM is often subjective and domain-specific.1,3
The most common unsupervised learning method is cluster analysis, which is used for exploratory data analysis to find hidden patterns or grouping in data. The clusters are modeled using a measure of similarity.
Unsupervised learning methods are used in bioinformatics for sequence analysis and genetic clustering, in data mining for sequence and pattern mining, in medical imaging for image segmentation, and in computer vision for object recognition.4 In medicine, currently, the ultimate MLM applied to image recognition are being developed for computed tomography (CT) scans and machine resonance imaging (MRI) image recognition for brain tumor diagnosis.5
Tree-Based Models (TBM)
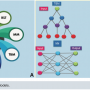
Based on a tree shape (Figure 1B), TBMs can be used to predict a possible outcome based on the input given. In Figure 1, the top black dots are the observations-input. The branch-splitting is the feature, and the final lower dots are the target.6 For example, in clinical thinking, if a patient with known comorbidities such as hypertension and smoking comes in with oppressive chest pain that radiates to the jaw, and the patient is diaphoretic, the physician can suspect the patient is having a myocardial infarction (MI). The same logic applies to TBMs used for prediction; the model is trained on data to learn the features and the strength of the relationships among them. Based on this information, the TBM can predict an outcome.
TBMs can also be used for classification.6 For example, with an inferior ST elevation myocardial infarction (STEMI) diagnosis, assume that in Figure 1B, the top black dot is the input ST segment from the derivations II, III, and aVF. The branches or divisions between inferior nodes can be the following: Is ST >0,1? Is ST elevation present in II and III? Is it present on III and aVF? Is ST elevation in all three leads? If the answer to all the above queries is a YES, then the output will be “inferior STEMI”. If the answer to the previous questions is a NO, then the output will be “non-inferior STEMI”. This is a simple example of how the TBM can be trained for hierarchy classification: multiple trees can be combined to assess different features and to make the MLM more precise.
Fuzzy Set Theories
Fuzzy set theories enable recognition of an element into a set, even if the element belongs only partially to that set, by taking into account that the total membership values add to one. It provides an effective way to approximate features. Fuzzy logic introduces partial truth values, between true and false.
According to Aristotelian logic, for a given proposition or state, we only have two logical values: true-false, black-white, 1-0. In real life, most things are not either black or white, but grey. Thus, in many practical situations, it is convenient to consider intermediate logical values. Take a very simple medical example: consider the statement “you are healthy”. Is it true if you have only a broken nail? Is it false if you have a terminal cancer? Everybody is healthy to some degree h and ill to some degree i. If you are totally healthy, then of course h = 1, i = 0.7
Rough Set Theory (RST) and Markov Model (MM)
RST is a type of reinforcement MLM that approximates a feature based on the fact that it belongs to a pair of sets. These sets give an upper and lower value in the original set, called crisp sets. This technique is used with a statistical model known as a Hidden Markov Model that allows determining through statistics parameters that are hidden in the data from observable parameters. RST assists in the process of developing rules that might be understood in an easy way by the system. In this way, it can lead to accurate extraction of important information that is previously shown in a complex way in the database, therefore improving the classification model. In healthcare, the Markov Model can be applied to medical decision-making sequences, perform cost-effective analysis of a procedure, and model the progression of chronic disease.8
Genetic Algorithms (GA)
Genetic algorithms are defined as a group of optimization algorithms that attempt to establish the best values to feed the system (input) in order to get the best results (output). This technique follows the principle of genetics. Results depend on the quality of the material with which the system is fed, similar to how the phenotype depends on genotype in human genetics.9 In healthcare, GA have been applied to image recognition in radiology, as well as treatment planning and pharmacovigilance.10 Most advances related to this AI are developing tools.
Neural Networks (NN)
As the name states, this type of machine learning theory was inspired by the connections between neurons in the nervous system and the proposed theories of learning. Neural Networks (NN) establishes relationships among features by interconnecting several basic units called neurons (Figure 1B, white circles in the model next to NN). The number of layers, represented by vertically stacked neurons in Figure 1B, represents the depth and type of relationships that the algorithm is capable of recognizing.11 Recurrent Neural Networks (RNN) and Convolutional Neural Networks (CNN) follow this path in pattern recognition. Currently, these two MLM are the most important to understand, as they are have perhaps the greatest application for medical data classification. Table 1 shows two articles describing the use of NN for ECG wave identification and the topographic diagnosis of MI. This type of deep learning has shown to have the best results in terms of accuracy.
Hybrid Algorithm
Hybrid algorithms are a combination of two or more systems that are employed in combination to solve a problem. This can be done by using either one technique or the other, or, by replacing one for the other along the development of the process. The hybrid algorithm allows a better functioning system than the one that would be achieved by using single technique, because MLMs complement each other to allow a superior overall performance. As an example, Table 1 features an article by Bensujin et al16 where a hybrid algorithm was used for STEMI diagnosis.
Relevant Research
 According to the Harvard Business Review 2018, the most promising healthcare advancements in AI could hugely change the healthcare delivery system (Figure 3). We have also assembled a review (Table 1) of the most relevant and recent publications in the field of AI applications in cardiology. Specifically, we have selected those that relate to acute myocardial infarction and STEMI. These diverse publications demonstrate the wide expanse of different machine learning applications being applied to cardiology that are related to the automation of ECG interpretation.
According to the Harvard Business Review 2018, the most promising healthcare advancements in AI could hugely change the healthcare delivery system (Figure 3). We have also assembled a review (Table 1) of the most relevant and recent publications in the field of AI applications in cardiology. Specifically, we have selected those that relate to acute myocardial infarction and STEMI. These diverse publications demonstrate the wide expanse of different machine learning applications being applied to cardiology that are related to the automation of ECG interpretation.
Comparing Cardiologists and Machine Learning (ML)
Darwin said, “It is not the strongest of the species that survive, nor the most intelligent, but the one most responsive to change.” The practice of medicine passes on to the next generation in a deliberate and well-defined path, and this practice has successfully navigated tremendous change through almost two centuries. Medicine is unique in its manner of passing on a combination of academic knowledge and clinical practice to the next generation of students and practitioners. It begins with the finest physicians becoming masters in their individual specialties, through the thorough acquisition of knowledge gained through their experiences over decades of clinical practice. Subsequently, these masters counsel the next generation of students that, in turn, benefit from the experiences of their mentors, and can even surpass them. In terms of AI and ML, the “years of experience” are represented in the data that is used as input to train the equipment to recognize patterns and classify the input.
If we look again at ECG as an example, we can see that by inserting the data into the machine learning process, the algorithm learns in minutes what took years for physicians to master. Herein lies the transformational possibilities of AI and ML as it applies to various aspects of cardiology that will parallel what we have seen with the dramatic shifts in ECG interpretation. In other areas of cardiology, in particular, diagnostic echocardiography procedures and imaging studies, the changes may be equally dramatic. A quick snapshot of the amazing changes being pioneered through applications of AI in cardiology was evident at the Siemens Healthineers display booth at the recently concluded TCT meeting, where an analysis of the entire cardiac life cycle management was methodically deconstructed with systematic destructions of existing nomenclature and algorithms.
 Figure 4 details the comparison between sensitivity and specificity in STEMI diagnosis between physicians and ML techniques. According to McCabe et al12 in 2013, interventional cardiologists, not unexpectedly, were the most specific for making a STEMI diagnosis and achieved 89% specificity. In contrast, ANN achieved 96% specificity for MI topographic diagnosis, according to Costa et al.13 For sensitivity analysis, ANN reported 92% rates in comparison with 70% achieved by emergency physicians and 70% by interventional cardiologists (Figure 4). AI application in ECG evaluation leads to higher accuracy and specificity, along with faster diagnosis. Faster diagnosis, in particular, translates into improved triage of patients by decreasing unnecessary transfers while improving outcomes and therefore, reducing costs.
Figure 4 details the comparison between sensitivity and specificity in STEMI diagnosis between physicians and ML techniques. According to McCabe et al12 in 2013, interventional cardiologists, not unexpectedly, were the most specific for making a STEMI diagnosis and achieved 89% specificity. In contrast, ANN achieved 96% specificity for MI topographic diagnosis, according to Costa et al.13 For sensitivity analysis, ANN reported 92% rates in comparison with 70% achieved by emergency physicians and 70% by interventional cardiologists (Figure 4). AI application in ECG evaluation leads to higher accuracy and specificity, along with faster diagnosis. Faster diagnosis, in particular, translates into improved triage of patients by decreasing unnecessary transfers while improving outcomes and therefore, reducing costs.
 Now, the bottom line: Does this mean that our jobs are at stake? Truthfully, yes. But do not panic. Not yet, and not all jobs. However, understand that first and foremost, all lofty definitions aside, artificial intelligence means eliminating the physician entirely out of the equation! To illustrate the penetration of AI/ML into cardiology, and to highlight how systems will be permanently altered, refer to Figure 5, which shows how STEMI systems will be totally transformed with AI/ML.
Now, the bottom line: Does this mean that our jobs are at stake? Truthfully, yes. But do not panic. Not yet, and not all jobs. However, understand that first and foremost, all lofty definitions aside, artificial intelligence means eliminating the physician entirely out of the equation! To illustrate the penetration of AI/ML into cardiology, and to highlight how systems will be permanently altered, refer to Figure 5, which shows how STEMI systems will be totally transformed with AI/ML.
There is indeed a genuine apprehension of cannibalization when we look at endeavors to advance AI and ML. The efficiency of AI and ML is best viewed as the total elimination of the human interface. If a CEO of an AI/ML organization were to allocate bonuses for performance, the top-earning data scientist would be the one who totally gets rid of the physician from all supervised and non-supervised processes. Despite the tremendous and obvious potential of AI/ML in medicine, we firmly believe that it is most physicians’ essential desire for self-preservation that is slowing the adoption of AI/ML in medicine.
However, our defensive mechanisms will disappoint us all, and do so in a dismal fashion. An analogy to this phenomenon is the fate of the electronic medical record (EMR)’s incorporation into medicine — itself a crude and unscientific AI/ML function. Despite most physicians hating EMR and resenting its adoption, physicians have grudgingly espoused the new systems — sadly, without even a clear demonstration of its benefits. However, unlike with EMR, we believe the introduction and acceptance of the advent of AI/ML into healthcare will occur with greater scientific validity, and hopefully, with more collaboration among the various stakeholders. Were that not to happen, indeed, a most turbulent phase lies ahead. It will become the responsibility of cardiologists and physicians in all fields to recognize the tasks that can be optimized by incorporating AI into everyday work, with the potential of creating more available time to discuss procedures and treatment options with our patients.
Our best advice is to avoid resisting the inevitable advancements of AI/ML and instead, look for prudent approaches aimed at understanding AI. Assimilating the ML methodologies described herein is a good start. Build upon your knowledge of AI and the techniques that specially pertain to your practice. Identify areas that complement and those that may enhance your productivity. We recommend viewing AI’s movement into healthcare as offering additional tools rather than hindrances. Fortunately, time is still on our side, as none of these systems are ready for prime time — yet. As clinicians, we must face the advancement of AI into healthcare with understanding and innovation, or we may risk annihilation.
References
- Maini V. Machine learning for humans. In: A beginner’s guide to AI/ML. Medium. 2017 Aug. Available online at: https://medium.com/machine-learning-for-humans/why-machine-learning-matters-6164faf1df12. Accessed November 8, 2018.
- Patel S. Chapter 2: SVM (support vector machine) — theory. In: Machine learning 101. Medium. 2017 May. Available online at: https://medium.com/machine-learning-101/chapter-2-svm-support-vector-machine-theory-f0812effc72. Accessed November 8, 2018.
- Brownlee J. Supervised and unsupervised machine learning algorithms. In: Understand machine learning algorithms. Machine Learning Mastery. 2016 Mar. Available online at: https://machinelearningmastery.com/supervised-and-unsupervised-machine-learning-algorithms. Accessed November 8, 2018.
- Unsupervised learning. MathWorks. Available online at: https://www.mathworks.com/discovery/unsupervised-learning.html. Accessed November 8, 2018.
- Erickson BJ, Korfiatis P, Akkus Z, Kline TL. Machine learning for medical imaging. Radiographics. 2017; 37(2): 505-515.
- Venables WN, Ripley BD. Modern applied statistics with S. 4th ed. New York, New York: Springer-Verlag New York, Inc.; 2002.
- Torres A, Nieto JJ. Fuzzy logic in medicine and bioinformatics. J Biomed Biotechnol. 2006; 2006(2): 91908.
- Komorowski M, Raffa J. Markov models and cost effectiveness analysis: applications in medical research. In: MIT Critical Data. Secondary Analysis of Electronic Health Records. Springer Open: 2016; 351-367. doi: 10.1007/978-3-319-43742-2_24.
- Roopa KC, Harish BS. A survey on various machine learning approaches for ECG analysis. Int J Comput Appl. 2017; 163(9): 25-33. doi:10.5120/ijca2017913737.
- Ghaheri A, Shoar S, Naderan M, Hoseini SS. The applications of genetic algorithms in medicine. Oman Med J. 2015; 30(6): 406-416.
- O’Shea K, Nash R. An introduction to convolutional neural networks. arXiv:1511.08458v2 [cs.NE] 2 Dec 2015.
- McCabe J, Armstrong E, Ku I, et al. Physician accuracy in interpreting potential ST-segment elevation myocardial infarction electrocardiograms. J Am Heart Assoc. 2013; 2(5): e000268-e000268. doi:10.1161/jaha.113.000268
- Costa C, Silva I, de Sousa R, et al. The association between reconstructed phase space and artificial neural networks for vectorcardiographic recognition of myocardial infarction. J Electrocardiol. 2018; 51(3): 443-449. doi:10.1016/j.jelectrocard.2018.02.001
- Abrishami H, Campbell M, Han C, et al. P-QRS-T localization in ECG using deep learning. International Conference IEEE EMBS on Biomedical & Health Informatics (BHI). March 4-7, 2018: Las Vegas, Nevada.
- Berikol GB, Yildiz O, Özcan IT. Diagnosis of acute coronary syndrome with a support vector machine. J Med Syst. 2016 Apr;40(4):84. doi: 10.1007/s10916-016-0432-6.
- Bensujin, Kezi Selva vijila C, Hubert C. Detection of ST segment elevation myocardial infarction (STEMI) using bacterial foraging optimization technique. International Journal of Engineering and Technology. 2014; 6: 1212-1223.
- Salem ABM, Revett K, El-Dahshan ESA, et al. Machine learning in electrocardiogram diagnosis. 2009 International Multiconference on Computer Science and Information Technology, Mragowo, 2009: 429-433. doi: 10.1109/IMCSIT.2009.5352689
The authors can be contacted via Sameer Mehta, MD, at sameer.lumenglobal@gmail.com.











