The Latest CLI Therapeutic Options
Disclosure: Dr. Mustapha reports he is a consultant for Bard, Covidien, Cordis, CSI, Spectranetics, Boston Scientific, Cook, and Terumo. Dr. Beasley reports he is a trainer for Abbott Vascular, Bard, Boston Scientific, Cook Medical, Cordis, Medtronic/Covidien, CSI, Endologix, Gore, Lombard Medical, and Spectranetics. Dr. Scott reports no conflicts of interest regarding the content herein.
Dr. J.A. Mustapha can be contacted at jihad.mustapha@metrogr.org.
Critical limb ischemia (CLI) therapy is in the domain of multi-specialty, as it should be. Without the valuable therapeutic input from differing sides, we would not have reached the current status of CLI therapy. It is essential that we continue an open-mind approach to the CLI patient and always offer the best therapeutic option for the presented lesion. As we are aware, no two CLI patients present with the same anatomical disease. We can all agree there is inflow and outflow disease that needs to be  treated. Some can be treated surgically, some can be treated via endovascular approach, and some can be treated via a hybrid approach. When you think of CLI therapy today, think of all the options available to provide the patient “the best option.”
treated. Some can be treated surgically, some can be treated via endovascular approach, and some can be treated via a hybrid approach. When you think of CLI therapy today, think of all the options available to provide the patient “the best option.”
Interview with Robert Beasley, MD
J.A. Mustapha, MD: How many CLI patients do you treat per week?
Robert Beasley, MD: I treat an average of 5-10 CLI patients per week.
J. Mustapha: Are you seeing an increasing influx of CLI patients into your practice?
R. Beasley: I am definitely seeing an increase in the number of CLI patients coming into my practice over the past few years.
J. Mustapha: Has the acuity of patients with CLI worsened or gotten better over the last 5 years?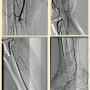
R. Beasley: My experience is that the acuity of patients with CLI has gotten better over the last 5 years, because of earlier diagnosis and better awareness of primary care providers and other referring physicians.
J. Mustapha: Do you see yourself doing more complex CLI procedures today compared to 5 years ago?
R. Beasley: I absolutely am doing many more complex CLI procedures now than 5 years ago.
J. Mustapha: What are some of the reasons that you are performing more interventions today?
R. Beasley: More community awareness, more patient awareness, and better primary care, internal medicine, and podiatry awareness. Also, an exponential improvement in treatment devices and technology allow for more successful complex procedures to be performed.
J. Mustapha: Do you believe new techniques and technologies have helped the outcome of your sick CLI patients?
R. Beasley: Yes. With the increase in successful complex procedures, I see much better outcomes for my sick CLI patients.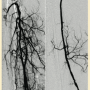
J. Mustapha: How often are you seeing patients as a second opinion after they have been told “nothing else can be done?”
R. Beasley: This is often the case. I am developing a rich referral network from many sources. We are becoming viewed as the place in Miami for CLI treatment.
J. Mustapha: If there is one technology you would love to see on the market in the United States for tibial treatment, what would it be?
R. Beasley: More microcatheters and sheaths for dedicated tibial interventions.
J. Mustapha: Do you believe drug-coated balloons are going to work in all CLI patients?
R. Beasley: Probably not all CLI patients — specifically, heavily calcified lesions present tremendous challenges.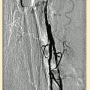
J. Mustapha: Are we doing enough for CLI awareness and amputation prevention?
R. Beasley: No, we are not — still today, in 2015, many patients are presenting to my office for a second or third opinion with advanced untreatable gangrene. We need to educate the community and promote intervention at a much earlier stage.
J. Mustapha: What advice do you have for those of us who perform CLI interventions to improve our overall outcomes?
R. Beasley: Never accept defeat (amputations) (see sidebar case). Always think outside the box for the next great approach/technique or technology.
Interview with Eric Scott, MD
J. Mustapha: How many CLI cases do you perform per week?
Eric Scott, MD: I perform 1-4 CLI cases per week. Approximately half of these patients are treated in an outpatient setting while the remaining half are hospitalized for concomitant treatment of infection, wounds, or medical co-morbidities.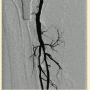
J. Mustapha: What attracted you to these types of complex procedures?
E. Scott: CLI patients challenge us in so many ways in the cath lab. Their disease is usually multi-level, chronic total occlusions (CTOs) are the norm, and these patients are older and sicker. They may come with significant renal impairment or be unable to even hold still well during imaging. Yet most of these patients can achieve limb salvage with good endovascular therapy alone if the operator is able to deliver it. These patients challenge me to learn new techniques, new devices, new wires, and they push me to be better at what I do each day. Ultimately, CLI patients still humble us all from time to time in the cath lab, a reminder that innovation in endovascular therapy must push on and that for some patients, surgical bypass remains an attractive option
J. Mustapha: Is it true that it can take anywhere between 3-5 hours to perform a tibial bypass?
E. Scott: Yes, or even longer. The gold standard for tibial bypass is a vein bypass, usually the greater saphenous vein. It is not uncommon, however, to have inadequate greater saphenous vein in the extremity in which you are performing the bypass. 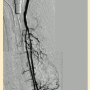 Harvesting vein from the contralateral leg or even an arm can extend operative times even longer.
Harvesting vein from the contralateral leg or even an arm can extend operative times even longer.
J. Mustapha: When was the last time you performed tibial bypass and how long did it take you?
E. Scott: I performed my last tibial bypass two weeks ago, actually two in one day. The first bypass was in a 72-year-old female with gangrene of toe. She had multiple levels of occlusion, a prosthetic knee joint, and no patent tibial arteries except for a patent common plantar artery at the ankle. She has limited greater saphenous vein, so I performed a bypass from the distal superficial femoral artery (SFA) to the common plantar artery. This required harvest of the saphenous vein from the groin distally, adding to the number of incisions required. A completion angiogram was performed and the entire procedure took about five hours. The second bypass was in a 15-year-old girl whose calf was severely injured in a motorcycle crash and her foot was acutely ischemic. Her proximal anterior tibial vessels were completely avulsed, and the tibioperoneal trunk and proximal peroneal and posterior tibial arteries were badly injured and thrombosed. I performed a popliteal to posterior tibial bypass with greater saphenous vein harvested from the contralateral thigh in two hours.
J. Mustapha: Personally, I am so glad tibial bypass is available. When should a CLI patient be referred for tibial bypass?
E. Scott: There are admittedly fewer and fewer tibial bypasses being performed anymore, but there are instances when it may be a superior option. I consider tibial bypass when faced with really long CTOs that involve multiple levels. If confronted with an angiogram that reveals severe common femoral disease and an SFA occlusion that extends through the popliteal artery and into the tibial arteries, I will usually stop and consider bypass options. Extreme cases like this usually require multiple devices, multiple accesses, and multiple hours. And you do it without the assurance of technical success. In contrast, I’ve never completed a tibial bypass and not achieved revascularization.
I also use tibial bypass for the occasional patient with early, recurrent occlusion following endovascular procedures. I have found that the toughest arterial occlusions to recanalize tend to be the segments that are hardest to keep open. The longer the diseased segment, the more troublesome segments there tend to be and the more vulnerable the entire segment is to re-occlusion. That is when a healthy, 4-5mm saphenous vein becomes a very appealing alternative conduit, especially if the patient can tolerate the operation.
J. Mustapha: Do you ever perform tibial bypass followed by pedal loop reconstruction in the same setting?
E. Scott: I haven’t. If a patient has such severe pedal disease that loop reconstruction is required, my inclination is to keep the intervention all endovascular. If a tibial bypass requires pedal loop reconstruction for sufficient outflow, I suspect most vascular surgeons, myself included, would argue it shouldn’t be performed in the first place. This would be infrequent, though. A vein bypass can stay open with surprisingly little outflow. And don’t forget that bypass outflow includes retrograde flow up the target vessel. I think the most appropriate use of this hybrid technique would be in the setting of tibial bypass to the “wrong” angiosome (an indirect revascularization) in patients with Rutherford 5 ischemia. In this setting, evidence shows our limb salvage rates are reduced in comparison to a “direct revascularization”. Predicting the failure of an indirect tibial bypass to successfully heal a wound is difficult though, as most bypasses do achieve limb salvage.
J. Mustapha: When doing hybrid CLI procedures, what are the most therapeutic combinations that you perform?
E. Scott: I honestly don’t perform many hybrid procedures for CLI, even though I have access to a hybrid operating room. The open surgical portion, as we discussed earlier, takes a substantial amount of time, and to add in additional layers of endovascular therapy can make for a really long operation and general anesthetic. If I’m doing it, though, it is usually a femoral endarterectomy or femoropopliteal bypass paired with an endovascular therapy for outflow. Sometimes the distal popliteal or tibioperoneal disease is more isolated than diffuse, and can easily be treated by endovascular therapies rather than long, tibial bypass.
J. Mustapha: Are you an angiosome-directed therapy believer?
E. Scott: I am, but I worry the concept has been oversimplified. Differing indications for intervention and heterogeneity of the pedal circulation are important variables that complicate decision-making. Patients with ischemic rest pain or diffuse digital  gangrene do not need an angiosome-based intervention. Patients with focal tissue loss do, but you have to understand that particular patient’s pedal circulation well to know how to get flow where they need it. I always get a magnified anteroposterior (AP) and lateral angiogram of the foot to best depict the state of direct and collateral circulation. I am amazed by how heterogeneous these circulations are in patients. The angiosome concept holds most true when there is a distinct absence of collaterals in the foot, often in the setting of diabetes mellitus. However, when there is a healthy collateral circulation via either an intact pedal arch or healthy peroneal artery with hypertrophied communicating branches, you can achieve equivalent revascularization via the “indirect” tibial artery with just as good a result. I think good pedal imaging often leads to the most appropriate intervention, more so than does a strict adherence to the angiosome concept.
gangrene do not need an angiosome-based intervention. Patients with focal tissue loss do, but you have to understand that particular patient’s pedal circulation well to know how to get flow where they need it. I always get a magnified anteroposterior (AP) and lateral angiogram of the foot to best depict the state of direct and collateral circulation. I am amazed by how heterogeneous these circulations are in patients. The angiosome concept holds most true when there is a distinct absence of collaterals in the foot, often in the setting of diabetes mellitus. However, when there is a healthy collateral circulation via either an intact pedal arch or healthy peroneal artery with hypertrophied communicating branches, you can achieve equivalent revascularization via the “indirect” tibial artery with just as good a result. I think good pedal imaging often leads to the most appropriate intervention, more so than does a strict adherence to the angiosome concept.
J. Mustapha: If a patient does not have a target vessel that feeds directly into the ischemic ulcer, would bypass be the next best option?
E. Scott: In this setting, I think endovascular therapies offer a distinct advantage. I can only bypass to one tibial artery, but I can easily try to open multiple tibial arteries to compensate for the lack of a “direct” revascularization. It is the most common indication for me to attempt intervention in multiple tibial arteries in a single setting.
J. Mustapha: What are your thoughts on venous arterialization for patients with end-stage CLI scheduled for major amputation?
E. Scott: I have never performed one of these procedures nor have I seen one. Published series do demonstrate some patients achieve limb salvage via the procedure, but it requires extensive venous valve destruction in the foot and additional pedal  incision to accomplish it. With today’s endovascular tools to enhance pedal arterial circulation, all without incising the foot or calf at all, I suspect the need for this approach is declining.
incision to accomplish it. With today’s endovascular tools to enhance pedal arterial circulation, all without incising the foot or calf at all, I suspect the need for this approach is declining.
J. Mustapha: As a vascular surgeon, do you perform more open or endovascular procedures for CLI therapy?
E. Scott: My endovascular procedures for CLI probably outnumber my surgical procedures by 10-15 to 1. I think there is a similar ratio of interventions in patients who have already required surgical bypass. Endovascular therapies routinely help me maintain patency of bypass grafts, either via intervention on the graft or its anastomoses, or by helping to maintain patency of the distal outflow.
J. Mustapha: Can you share a case where you treated both inflow and outflow in the same setting?
E. Scott: Last week I encountered an 80-year-old woman who was receiving inpatient treatment for a protracted pneumonia, further complicated by worsening rest pain and multiple ulcerations of her right toes. An arterial duplex demonstrated an occlusion of her right popliteal artery with evidence of additional tibial occlusive disease. Her ankle-brachial index was 0.38. I waited for her pneumonia to clear and then proceeded with formal angiography and intervention (Figure 1). Her initial angiographic images depicted a not-infrequent situation encountered in CLI patients — the absence of a healthy distal bypass target. Her dorsalis pedis artery was occluded, as was the common plantar artery. A short segment of the lateral plantar artery was identified in the foot that suggested this was likely the healthiest of the major pedal arteries.
I crossed the popliteal occlusion with a V-18 wire (Boston Scientific) and selected the posterior tibial artery with it. A 4mm Spider wire (Medtronic) was then positioned in the mid posterior tibial artery. I usually use some form of atherectomy in severely diseased popliteal arteries to minimize the risk of dissection and chose directional atherectomy (TurboHawk SX-C, Medtronic) for this lesion. Plain balloon angioplasty (2.5mm) was used for treatment of the distal posterior tibial lesions and the common plantar artery. A 2.0mm balloon was used for treatment of the lateral plantar artery. I completed the therapy with use of a 4mm drug-coated angioplasty balloon in the popliteal artery due to my perception of a high risk of restenosis in this segment.
Case: Averting a BTK Amputation
Robert E. Beasley, MD, Director, Vascular/Interventional Radiology Lab and Evanescence Vein Center, Mount Sinai Medical Center, Miami, Florida
Patient history
This is an 80-year-old male with history of diabetes, hypertension, hyperlipidemia, severe rest pain, and non-healing ulcers of the right lower extremity. The patient was told by 2 surgeons that he needed a below-the-knee (BTK) amputation; he was referred to us by a podiatrist.
Procedure
Peripheral angiography showed total occlusion of the right distal superficial femoral artery extending into the popliteal artery and into the trifurcation, with reconstitution of a peroneal and a posterior tibial. We attempted to obtain antegrade access to the lesions of the right infra-popliteal vessels (Figure 1). After failing to cross the lesion, we obtained retrograde access, puncturing from below the lesion through the posterior tibial artery near the ankle joint (Figure 2). Access was obtained and the lesion below the knee was crossed (Figure 3). Following this, the V-18 control wire (Boston Scientific) was snared from above, a CXI support catheter (CSI) was backloaded over the wire, and an antegrade Viperwire (CSI) was then placed, with removal of the retrograde sheath and wire. Atherectomy was performed with the 1.5mm Stealth 360° orbital atherectomy device (CSI) followed by balloon angioplasty with Cook, Sterling (Boston Scientific), and Chocolate (Cordis) balloon catheters. After angioplasty, significant improvement in luminal gain was noted at the expense of a dissection (Figure 4). We chose to place a Zilver PTX drug-eluting stent (Cook) within the distal superficial femoral and popliteal artery segment (Figure 5). After the procedure, the patient’s wounds completely healed. An angiogram 3 months later was performed, showing the vessels to be widely patent (Figure 6). The patient sent us a video of himself finally walking, and without pain (Video 1; online).










