Improving Patient Care and Post Procedure Efficiency Following Transradial Access

Over the past decade, transradial access (TRA) for invasive cardiac catheterization procedures has gained significant popularity in Europe, Canada, South America, Japan, and other sites outside of the United States where TRA is used in more than 60% of cases.1 The most compelling reason for adopting TRA is the increased patient safety that results from the potential elimination of access site bleeding and vascular complications. In addition, TRA is associated with early sheath removal, improved patient comfort, faster recovery, and lower costs in comparison with transfemoral access.2-4 In the United States, TRA catheterization procedures are gaining significant momentum, with growth from 2% in 2007 to approximately 15% in 2011, and an estimated 30-35% in 2016.5 Moreover, with increased availability of training opportunities, the TRA utilization rate is expected to grow to 50% or more in the next 2-4 years.
While transitioning to a primary transradial operator and establishing a transradial program can initially seem to be formidable tasks, there are plethora of resources to help the operator learn the skill set needed to make this transition successful. Programs, such as the Society for Cardiovascular Angiography and Interventions (SCAI) Transradial Intervention Program (TRIP) program, provide not only physician education, but also staff education focused on appropriate cath lab setup and peri-procedural care. Unfortunately, lack of education for hospital staff outside of the cath lab and its post procedure area can be a significant barrier to the growth of a successful transradial program and is often unrecognized. Although hospital systems are quick to embrace the transradial approach due to its proven cost savings, improved outcomes, and an increase in same-day discharge, only a few have dedicated the resources to streamlining recovery post-TRA through dedicated transradial recovery areas or radial lounges. When these areas do exist, they are often limited in size and capacity. Alternatively, recovery post-TRA can be successfully transitioned to inpatient areas, but implementing this strategy can be challenging. In our experience, these challenges may be more frequent in larger hospitals, where staffing patterns, including the presence of a central staffing structure and frequent nurse travelers, make it difficult to ensure consistency of inpatient nursing staff that is experienced with hemostasis protocols and access site management. Additionally, increasing nurse to patient ratios prevent nurses from being able to manage intensive protocols that require frequent assessments and hemostasis band adjustments.
Although these post procedure obstacles can be successfully overcome, it is a work-intensive process that requires an ongoing commitment from physician and nursing leaders, as well as hospital administrative staff. Not surprisingly, an informal poll of the #RadialFirst group on Twitter suggested that a significant percentage of operators still have their patients recover in a post procedure unit. When this is the case, the capacity of the post procedure recovery unit can be the rate-limiting step to procedure volume and program growth. In addition, the post cath recovery of late cases can lead to increased overtime costs and a deterioration of job satisfaction among staff. The recovery of emergent cases during nights and weekends can also present a logistical challenge, requiring the patient to be recovered by either cath lab staff or nursing staff in intermediate or intensive care units to allow for the appropriate nurse-to-patient ratio due to time-intensive hemostasis protocols. This results in tremendous inefficiency and unneeded resource utilization, particularly in cases where patients do not require this higher level of care.
Introduction of StatSeal Advanced
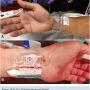 Facing some of the challenges described above was the primary motivation behind a trial of the StatSeal Advanced (Biolife) at North Florida Regional Medical Center and The Cardiac and Vascular Institute in Gainesville, Florida. The StatSeal Advanced is a disc comprised of a topical hydrophilic polymer and potassium ferrate by compression of the compound during manufacturing (Figure 1A-B). When in direct contact with even a small amount of blood, the compound forms an occlusive seal through simultaneous iron-mediated agglomeration of blood solids/proteins and rapid dehydration of the blood. This seal formation is independent of the clotting cascade, facilitating sealing regardless of the anticoagulation/antiplatelet status of the patient. Introduced in 2015, the use of this compound in conjunction with commercially available hemostasis bands has demonstrated the potential to significantly shorten the time required to achieve hemostasis.6,7
Facing some of the challenges described above was the primary motivation behind a trial of the StatSeal Advanced (Biolife) at North Florida Regional Medical Center and The Cardiac and Vascular Institute in Gainesville, Florida. The StatSeal Advanced is a disc comprised of a topical hydrophilic polymer and potassium ferrate by compression of the compound during manufacturing (Figure 1A-B). When in direct contact with even a small amount of blood, the compound forms an occlusive seal through simultaneous iron-mediated agglomeration of blood solids/proteins and rapid dehydration of the blood. This seal formation is independent of the clotting cascade, facilitating sealing regardless of the anticoagulation/antiplatelet status of the patient. Introduced in 2015, the use of this compound in conjunction with commercially available hemostasis bands has demonstrated the potential to significantly shorten the time required to achieve hemostasis.6,7
 By introducing the protocol (Figure 2) developed at the University of Miami by Mauricio Cohen, MD, to our facilities, we were able to reduce post cath recovery time with no increase in bleeding rates. The protocol, which requires two deflations, proved to be easier and less demanding for the post cath recovery unit to follow when compared to our previous protocol that required multiple band adjustments at short time intervals. By decreasing the time to hemostasis and simplifying the protocol, we were able to reduce time to discharge, and quickly increase bed and staff availability in the recovery unit, allowing for greater capacity. The protocol also resulted in improved nursing staff satisfaction due to decreased time spent on management of intensive hemostasis protocols and late recovery of patients. Reida Squires, director of the Cardiovascular Center at North Florida Regional Medical Center shared that “Both the cath lab and recovery area staff always appreciated the advantages of the radial approach for our patients, but did find the recovery process time intensive. The establishment of the new hemostasis protocol in our lab has been helpful by not only allowing us to increase the growth of our transradial program, but also by leading to the program being enthusiastically embraced by our staff.”
By introducing the protocol (Figure 2) developed at the University of Miami by Mauricio Cohen, MD, to our facilities, we were able to reduce post cath recovery time with no increase in bleeding rates. The protocol, which requires two deflations, proved to be easier and less demanding for the post cath recovery unit to follow when compared to our previous protocol that required multiple band adjustments at short time intervals. By decreasing the time to hemostasis and simplifying the protocol, we were able to reduce time to discharge, and quickly increase bed and staff availability in the recovery unit, allowing for greater capacity. The protocol also resulted in improved nursing staff satisfaction due to decreased time spent on management of intensive hemostasis protocols and late recovery of patients. Reida Squires, director of the Cardiovascular Center at North Florida Regional Medical Center shared that “Both the cath lab and recovery area staff always appreciated the advantages of the radial approach for our patients, but did find the recovery process time intensive. The establishment of the new hemostasis protocol in our lab has been helpful by not only allowing us to increase the growth of our transradial program, but also by leading to the program being enthusiastically embraced by our staff.”
The implementation of this simplified protocol will also allow us to revisit transferring the recovery of these patients to the inpatient setting, since access site management will now require less nurse training and no increase in the nurse-to-patient ratio.
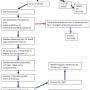 Anecdotally, the use of the StatSeal Advanced in our center, as well as other facilities, does not appear to have resulted in higher rates of radial artery occlusion (RAO). This may be explained by the reduction of compression time, as radial artery occlusion appears to be a function of both time and pressure required for hemostasis. Still, the issue of radial artery occlusion using this device is an important question that has not been systematically studied and remained a concern of our operators. In keeping with best practices, we introduced a modified protocol incorporating patent hemostasis, which also allowed us to further simplify the deflation protocol, with no increase in time to hemostasis. To validate this approach, we have started enrollment in a physician-initiated, prospective, single-arm observational pilot study to evaluate the incidence of radial artery complications (RAO, bleeding, hematoma) using the StatSeal Advanced disc and a modified protocol designed to simplify, standardize, and minimize clinician manipulation of hemostasis bands after initial application to the patient (Figure 3). For all patients, radial artery patency will be evaluated by ultrasound prior to discharge.
Anecdotally, the use of the StatSeal Advanced in our center, as well as other facilities, does not appear to have resulted in higher rates of radial artery occlusion (RAO). This may be explained by the reduction of compression time, as radial artery occlusion appears to be a function of both time and pressure required for hemostasis. Still, the issue of radial artery occlusion using this device is an important question that has not been systematically studied and remained a concern of our operators. In keeping with best practices, we introduced a modified protocol incorporating patent hemostasis, which also allowed us to further simplify the deflation protocol, with no increase in time to hemostasis. To validate this approach, we have started enrollment in a physician-initiated, prospective, single-arm observational pilot study to evaluate the incidence of radial artery complications (RAO, bleeding, hematoma) using the StatSeal Advanced disc and a modified protocol designed to simplify, standardize, and minimize clinician manipulation of hemostasis bands after initial application to the patient (Figure 3). For all patients, radial artery patency will be evaluated by ultrasound prior to discharge.
Conclusion
Building a successful transradial program requires clinical and programmatic changes that are sometimes challenging and overlooked. At our facility, the Cohen protocol using the StatSeal Advanced has been effective at decreasing post procedure recovery time with no increase in bleeding, also simplifying the post procedure protocol to allow for improved staff efficiency. The rate of RAO using this technique appears to be low, but needs to be more extensively examined. Several studies are currently underway, including one at our institution evaluating a modified protocol using the practice of patent hemostasis.
References
- Rao SV, Cohen MG, Kandzari DE, Bertrand OF, Gilchrist IC. The transradial approach to percutaneous coronary intervention: historical perspective, current concepts, and future directions. J Am Coll Cardiol. 2010; 55: 2187-2195.
- Cooper CJ, El-Shiekh RA, Cohen DJ, Blaesing L, Burket MW, Basu A, Moore JA. Effect of transradial access on quality of life and cost of cardiac catheterization: A randomized comparison. Am Heart J. 1999; 138: 430-436.
- Jolly SS, Amlani S, Hamon M, Yusuf S, Mehta SR. Radial versus femoral access for coronary angiography or intervention and the impact on major bleeding and ischemic events: a systematic review and meta-analysis of randomized trials. Am Heart J. 2009; 157: 132-140.
- Chase AJ, Fretz EB, Warburton WP, Klinke WP, Carere RG, Pi D, Berry B, Hilton JD. Association of the arterial access site at angioplasty with transfusion and mortality: the M.O.R.T.A.L study (Mortality benefit Of Reduced Transfusion after percutaneous coronary intervention via the Arm or Leg). Heart. 2008; 94: 1019-1025.
- Safley DM, Amin AP, House JA, Baklanov D, Mills R, Giersiefen H, Bremer A, Marso SP. Comparison of costs between transradial and transfemoral percutaneous coronary intervention: a cohort analysis from the Premier research database. Am Heart J. 2013; 165: 303-309 e302.
- Wang DS, Chu LF, Olson SE, Miller FJ, Valji K, Wong WH, Rose SC, Austin M, Kuo MD. Comparative evaluation of noninvasive compression adjuncts for hemostasis in percutaneous arterial, venous, and arteriovenous dialysis procedures, access procedures. J Vasc Interv Radiol. 2008 Jan; 19(1): 72-79. doi: 10.1016/j.jvir.2007.08.028.
- Rollefson W, Nash G, Cilingiroglu M, Mego D. Utilization of a potassium ferrate (K2FeO4) hemostatic disc (StatSeal™) to accelerate time to hemostasis in transradial cardiac procedures (TRA). Abstract SCAI 2016; Presented May 2016. Available online at https://statseal.com/wp-content/uploads/2016/10/SCAI-Rollefson-poster-5-16.pdf. Accessed June 21, 2017.
Disclosure: Matheen A. Khuddus, MD, FACC, FSCAI, reports institutional research grant support from Biolife and membership on the Medtronic Transradial Medical Advisory board.
Matheen Khuddus can be reached at mkhuddus@tcavi.com or on Twitter @matheenkhuddus.










