Critical Limb Ischemia: Revascularization Techniques to Optimize Endovascular Therapy in Complex Cases Using the JETSTREAM Atherectomy System
Introduction
The prevalence of peripheral vascular disease (PVD) has been increasing worldwide. The number of patients suffering from PVD is expected to increase by 15% in western countries and 30% in developing countries.1 This increase is a reflection of other co-morbidities driving the rise in numbers. Patients with PVD suffer from higher morbidity and mortality.2,3 Multiple modalities exist to evaluate and treat patients with PVD. Endovascular revascularization of patients with PVD is becoming a front-line strategy adopted by several disciplines, including vascular surgery, radiology, and cardiology. Bypass surgery is an excellent procedure in appropriately selected patients.4,5 However, many PVD patients may not be adequate candidates for surgical bypass. Endovascular therapy has been fueled by continuous innovation in techniques and devices.6 The JETSTREAM™ Atherectomy System (Boston Scientific) is a rotational device, featuring front-cutting, expandable blades. JETSTREAM is also the only atherectomy system with active aspiration. The rotational aspect of the device allows for plaque modification and debulking. The device is engineered to predictably treat multiple morphologies, such as calcium, plaque or thrombus, commonly found in total occlusions. We will present a case that demonstrates the versatility of the device in treating a complex clinical and anatomical situation in a patient with critical limb ischemia (CLI).
Case presentation
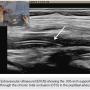
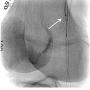
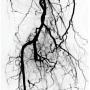
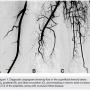 A 54-year-old male originally presented with complaints of life-limiting claudication, placing him at Rutherford class III. The patient’s past medical history was significant for hypertension and diabetes. He underwent a bio-prosthetic aortic valve replacement five years prior. There were no other significant co-morbidities. His clinical exam revealed a cold right lower extremity with no palpable pulses. The clinical workup included a 2-dimensional echo with an ankle-brachial index (ABI). Echo revealed his left ventricular function to be preserved, with no valvular or wall motion abnormalities. The right-side ABI was significantly abnormal at 0.4. The patient did not have any history of deep venous thrombosis or pulmonary embolism, nor was there any history of arterial thrombosis. A diagnostic angiogram revealed evidence of multi-level disease involving the right lower extremity. The lesion appeared to extend from the distal popliteal artery into the posterior tibial and tibio-peroneal trunks. The patient had an anomalous high take-off of the right posterior tibial (Figure 1).
A 54-year-old male originally presented with complaints of life-limiting claudication, placing him at Rutherford class III. The patient’s past medical history was significant for hypertension and diabetes. He underwent a bio-prosthetic aortic valve replacement five years prior. There were no other significant co-morbidities. His clinical exam revealed a cold right lower extremity with no palpable pulses. The clinical workup included a 2-dimensional echo with an ankle-brachial index (ABI). Echo revealed his left ventricular function to be preserved, with no valvular or wall motion abnormalities. The right-side ABI was significantly abnormal at 0.4. The patient did not have any history of deep venous thrombosis or pulmonary embolism, nor was there any history of arterial thrombosis. A diagnostic angiogram revealed evidence of multi-level disease involving the right lower extremity. The lesion appeared to extend from the distal popliteal artery into the posterior tibial and tibio-peroneal trunks. The patient had an anomalous high take-off of the right posterior tibial (Figure 1).
Clinical course
Due to the complex nature of this occlusion, therapy options included medical therapy, single-vessel tibial bypass, or endovascular therapy. The lesion involved the trifurcation of all tibial vessels where the distal posterior tibial artery was the only runoff to the foot. After discussing the options with our patient, the decision was made to attempt medical therapy. The patient was placed on antiplatelet therapy with aspirin. Aggressive lipid-lowering therapy was also initiated. The patient was not able to tolerate cilostazol. He continued with a self-administered walking program. In addition, we placed the patient on anticoagulation with warfarin in an attempt to treat what we felt was a thrombotic component on top of the atherosclerotic disease. Despite treating the patient for 3 months with the above-mentioned regimen, the patient’s symptoms continued to worsen. The patient started complaining of intermittent rest pain, placing him at Rutherford class IV. At this point, we decided to proceed with endovascular therapy in an effort to preserve tibial vessels.
Revascularization steps
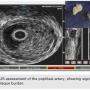
 Access. For any intervention requiring the crossing of a chronic total occlusion (CTO) or treatment of tibial vessels, it is our opinion that operators should strongly consider antegrade access. This case required multi-vessel, multi-level intervention, and antegrade access afforded the operator superior pushability, trackability, and torquability. We use ultrasound (US) guidance in gaining access to any vascular conduit. We started with a 5 French Pinnacle Precision sheath (Terumo) and after crossing, upsized to a 7 French Destination Pinnacle 45-centimeter sheath (Terumo).
Access. For any intervention requiring the crossing of a chronic total occlusion (CTO) or treatment of tibial vessels, it is our opinion that operators should strongly consider antegrade access. This case required multi-vessel, multi-level intervention, and antegrade access afforded the operator superior pushability, trackability, and torquability. We use ultrasound (US) guidance in gaining access to any vascular conduit. We started with a 5 French Pinnacle Precision sheath (Terumo) and after crossing, upsized to a 7 French Destination Pinnacle 45-centimeter sheath (Terumo).
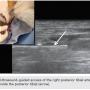
CTO crossing. The use of ultrasound (US) in gaining access to any vascular bed has been shown to be safe and effective. In addition, it is our experience that extra vascular US (EVUS) can also guide CTO crossing and therapy delivery. In this case, a .035-inch Navicross catheter (Terumo) was used to advance through the CTO in a rotational fashion. EVUS allowed us to guide the catheter and navigate the CTO (Figure 2). We were successful in crossing the CTO in the popliteal and peroneal arteries. Once crossing to the distal peroneal was confirmed (Figure 3), we started preparing for therapy.
 Lesion assessment. There are multiple modalities beyond angiography that permit the operator to evaluate the nature of the atherosclerotic disease. In this case, we utilized EVUS and intravascular US (IVUS). The IVUS catheter (Volcano Corporation) was advanced to the distal peroneal artery. An automated pullback is preferred to ensure that no segments within the vessel are missed. The IVUS image revealed evidence of significant hypo-echogenic plaque burden extending from the peroneal to the popliteal artery (Figure 4). EVUS images provided similar information. The hypo-echogenic appearance suggested soft plaque. The JETSTREAM System features active aspiration, making it well-suited for the thrombotic-component of this lesion. Its rotational blades also provide plaque modification and debulking, making JETSTREAM an ideal solution for this type of lesion morphology.
Lesion assessment. There are multiple modalities beyond angiography that permit the operator to evaluate the nature of the atherosclerotic disease. In this case, we utilized EVUS and intravascular US (IVUS). The IVUS catheter (Volcano Corporation) was advanced to the distal peroneal artery. An automated pullback is preferred to ensure that no segments within the vessel are missed. The IVUS image revealed evidence of significant hypo-echogenic plaque burden extending from the peroneal to the popliteal artery (Figure 4). EVUS images provided similar information. The hypo-echogenic appearance suggested soft plaque. The JETSTREAM System features active aspiration, making it well-suited for the thrombotic-component of this lesion. Its rotational blades also provide plaque modification and debulking, making JETSTREAM an ideal solution for this type of lesion morphology.
Alternative access. The nature of the occlusion in this case highlights the necessity of alternative access. The lesion extended from the popliteal into the posterior tibial/tibio-peroneal trunk/peroneal arteries. The angiogram showed a flush occlusion of the posterior tibial with reconstitution. Retrograde posterior tibial US-guided access was the only option to gain access into the distal patent portion (Figure 5). We placed a low-profile Cook tibiopedal sheath (2.9 French) (Cook Medical). Using an .018-inch CXI support catheter (Cook Medical), we advanced an .014-inch Glidewire Advantage wire (Terumo). Crossing into the true lumen of the popliteal artery in a retrograde fashion was relatively simple. Using a 7 mm snare, we were able to trap the Glidewire Advantage wire and reverse access (Figure 6). After reversing access, the wire in the posterior tibial artery was now flossed through the sheath. The posterior tibial sheath acted as an embolic protection system. The peroneal artery CTO was crossed in an antegrade fashion and the wire was placed in the distal peroneal artery. An embolic protection device was deployed in the distal peroneal artery. In an effort to protect the distal posterior tibial, we accessed the posterior tibial and crossed the occlusion after performing atherectomy of the popliteal artery. In addition, we did not want to use the larger JETSTREAM device in the popliteal while there was another wire present.
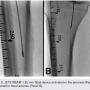
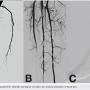
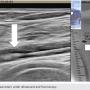 Atherectomy with JETSTREAM. Atherectomy was performed in two stages. During the first stage, we had wire access from the popliteal to the peroneal artery. Before crossing from the posterior tibial, we wanted to treat the popliteal/peroneal segment. A 2.4 mm/3.4 mm JETSTREAM atherectomy device was advanced into the popliteal and activated. The device must be activated with the blades down. A pilot channel will remove some of the plaque. The device is advanced at a rate of no more than 1 mm per second. The operator will need to listen to the pitch of the device. A depressed pitch suggests that the device rotation has slowed down, and the operator should then carefully pull the device back and re-advance. The operator must not allow the aspirational capabilities of the device to be overwhelmed. After successful atherectomy “blades-down,” a “blades-up” run was then performed with the JETSTREAM device (Figure 7). During the second stage of atherectomy, the peroneal and posterior tibial arteries required further therapy. For this stage, we obtained posterior tibial access, crossed the posterior tibial CTO, and snared the posterior tibial wire. We proceeded with the use of a 1.85 mm JETSTREAM device. The tibial device has the rotational capabilities without the “blades-up” feature, and also features active aspiration, like the larger JETSTREAM devices. We were able to atherectomize the posterior tibial and peroneal arteries (Figure 8). Angiographic images post atherectomy demonstrate significant plaque reduction. After atherectomy, we proceeded with kissing balloon angioplasty of the popliteal/peroneal and posterior tibial arteries (Figure 9). Angiography of the popliteal artery post atherectomy showed significant improvement (Figure 10). After removal of the pedal sheath, brisk flow was noted (Figure 11). The procedure was concluded with the resolution of the stenosis from the popliteal artery and restoration of flow in two tibial vessels (Figure 12).
Atherectomy with JETSTREAM. Atherectomy was performed in two stages. During the first stage, we had wire access from the popliteal to the peroneal artery. Before crossing from the posterior tibial, we wanted to treat the popliteal/peroneal segment. A 2.4 mm/3.4 mm JETSTREAM atherectomy device was advanced into the popliteal and activated. The device must be activated with the blades down. A pilot channel will remove some of the plaque. The device is advanced at a rate of no more than 1 mm per second. The operator will need to listen to the pitch of the device. A depressed pitch suggests that the device rotation has slowed down, and the operator should then carefully pull the device back and re-advance. The operator must not allow the aspirational capabilities of the device to be overwhelmed. After successful atherectomy “blades-down,” a “blades-up” run was then performed with the JETSTREAM device (Figure 7). During the second stage of atherectomy, the peroneal and posterior tibial arteries required further therapy. For this stage, we obtained posterior tibial access, crossed the posterior tibial CTO, and snared the posterior tibial wire. We proceeded with the use of a 1.85 mm JETSTREAM device. The tibial device has the rotational capabilities without the “blades-up” feature, and also features active aspiration, like the larger JETSTREAM devices. We were able to atherectomize the posterior tibial and peroneal arteries (Figure 8). Angiographic images post atherectomy demonstrate significant plaque reduction. After atherectomy, we proceeded with kissing balloon angioplasty of the popliteal/peroneal and posterior tibial arteries (Figure 9). Angiography of the popliteal artery post atherectomy showed significant improvement (Figure 10). After removal of the pedal sheath, brisk flow was noted (Figure 11). The procedure was concluded with the resolution of the stenosis from the popliteal artery and restoration of flow in two tibial vessels (Figure 12).
Clinical follow-up. The patient was discharged home the next day. On 30-day follow-up, the patient reported complete resolution of his symptoms. ABI was normalized at 1. The patient was maintained on dual antiplatelet therapy with aspirin and clopidogrel.
Discussion
 CLI represents the terminal stage of PVD and occurs when the capillary beds are inadequately perfused and unable to sustain tissue viability. CLI is defined by the presence of rest pain and/or tissue loss for at least 2 to 4 weeks that can be attributed to occlusive arterial disease. The diagnosis is clinical in nature and is classified as Fontaine stages III or IV, or Rutherford classification 4, 5, or 6.
CLI represents the terminal stage of PVD and occurs when the capillary beds are inadequately perfused and unable to sustain tissue viability. CLI is defined by the presence of rest pain and/or tissue loss for at least 2 to 4 weeks that can be attributed to occlusive arterial disease. The diagnosis is clinical in nature and is classified as Fontaine stages III or IV, or Rutherford classification 4, 5, or 6.
Anatomically, CLI is characterized by multi-level and multi-vessel, infrainguinal and tibial-pedal arterial stenosis and occlusions, compromising viability and threatening limb loss. It is estimated that 1.5 million people in Europe and 2 million people in the United States over age 50 manifest symptoms of CLI. One-year mortality and major amputation rates range from 20% to 50%.7-9 CLI occurs in approximately 1-3% of all PVD cases.10-12
 Atherectomy featuring active aspiration offers multiple advantages in treating atherosclerotic disease. JETSTREAM is the only atherectomy system offering active aspiration combined with plaque modification and debulking. The device can treat multiple morphologies such as calcium, plaque, or thrombus. The front-cutting expandable blades are designed to deliver concentric lumens. In addition, active aspiration is occurring at the same time as use, helping minimize the risk of distal embolization. Zeller et al showed its safety and efficacy in treating PVD in different vascular beds. The JETSTREAM device was successful in treating 99% of lesions. The rate of target vessel revascularization was 26% in lesions as long as 10 cm.13 The device was recently evaluated in calcified vessels. IVUS assessment showed the JETSTREAM atherectomy system removed and modified severe to moderate superficial calcium to achieve significant lumen gain. Adjunctive balloon angioplasty showed further lumen increase without major complications.14
Atherectomy featuring active aspiration offers multiple advantages in treating atherosclerotic disease. JETSTREAM is the only atherectomy system offering active aspiration combined with plaque modification and debulking. The device can treat multiple morphologies such as calcium, plaque, or thrombus. The front-cutting expandable blades are designed to deliver concentric lumens. In addition, active aspiration is occurring at the same time as use, helping minimize the risk of distal embolization. Zeller et al showed its safety and efficacy in treating PVD in different vascular beds. The JETSTREAM device was successful in treating 99% of lesions. The rate of target vessel revascularization was 26% in lesions as long as 10 cm.13 The device was recently evaluated in calcified vessels. IVUS assessment showed the JETSTREAM atherectomy system removed and modified severe to moderate superficial calcium to achieve significant lumen gain. Adjunctive balloon angioplasty showed further lumen increase without major complications.14
Conclusion
PVD is an epidemic that impacts a large number of patients worldwide. The disease is complex in nature, with multi-vessel and multi-level disease. The JETSTREAM atherectomy system is designed to allow clinicians to treat a variety of morphologies, including calcium, plaque or thrombus.The current body of evidence shows a high rate of success with a low rate of complications.
References
- Fowkes FG, Rudan D, Rudan I, Aboyans V, Denenberg JO, McDermott MM, et al. Comparison of global estimates of prevalence and risk factors for peripheral artery disease in 2000 and 2010: a systematic review and analysis. Lancet. 2013; 382(9901): 1329-1340.
- Patel MR, Conte MS, Cutlip DE, Dib N, Geraghty P, Gray W, et al. Evaluation and treatment of patients with lower extremity peripheral artery disease: consensus definitions from Peripheral Academic Research Consortium (PARC). J Am Coll Cardiol. 2015; 65(9): 931-941.
- Rooke TW, Hirsch AT, Misra S, Sidawy AN, Beckman JA, Findeiss L, et al. Management of patients with peripheral artery disease (compilation of 2005 and 2011 ACCF/AHA Guideline Recommendations): a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2013; 61(14): 1555-1570.
- Leather RP, Shah DM, Chang BB, Kaufman JL. Resurrection of the in situ saphenous vein bypass. 1000 cases later. Ann Surg. 1988; 208(4): 435-442.
- Taylor LM, Jr., Edwards JM, Porter JM. Present status of reversed vein bypass grafting: five-year results of a modern series. J Vasc Surg. 1990; 11(2): 193-205; discussion 205-206.
- Jaff MR, White CJ, Hiatt WR, Fowkes GR, Dormandy J, Razavi M, et al. An update on methods for revascularization and expansion of the TASC lesion classification to include below-the-knee arteries: a supplement to the Inter-Society Consensus for the Management of Peripheral Arterial Disease (TASC II): The TASC Steering Comittee. Ann Vasc Dis. 2015; 8(4): 343-357.
- Bosiers M, Scheinert D, Peeters P, Torsello G, Zeller T, Deloose K, et al. Randomized comparison of everolimus-eluting versus bare-metal stents in patients with critical limb ischemia and infrapopliteal arterial occlusive disease. J Vasc Surg. 2012; 55(2): 390-398.
- Balzer JO, Zeller T, Rastan A, Sixt S, Vogl TJ, Lehnert T, et al. Percutaneous interventions below the knee in patients with critical limb ischemia using drug eluting stents. J Cardiovasc Surg (Torino). 2010; 51(2):183-191.
- Siablis D, Karnabatidis D, Katsanos K, Diamantopoulos A, Christeas N, Kagadis GC. Infrapopliteal application of paclitaxel-eluting stents for critical limb ischemia: midterm angiographic and clinical results. J Vasc Interv Radiol. 2007; 18(11): 1351-1361.
- Lambert MA, Belch JJ. Medical management of critical limb ischaemia: where do we stand today? J Intern Med. 2013; 274(4): 295-307.
- Hirsch AT, Haskal ZJ, Hertzer NR, Bakal CW, Creager MA, Halperin JL, et al. ACC/AHA 2005 guidelines for the management of patients with peripheral arterial disease (lower extremity, renal, mesenteric, and abdominal aortic): executive summary a collaborative report from the American Association for Vascular Surgery/Society for Vascular Surgery, Society for Cardiovascular Angiography and Interventions, Society for Vascular Medicine and Biology, Society of Interventional Radiology, and the ACC/AHA Task Force on Practice Guidelines (Writing Committee to Develop Guidelines for the Management of Patients With Peripheral Arterial Disease) endorsed by the American Association of Cardiovascular and Pulmonary Rehabilitation; National Heart, Lung, and Blood Institute; Society for Vascular Nursing; TransAtlantic Inter-Society Consensus; and Vascular Disease Foundation. J Am Coll Cardiol. 2006; 47(6): 1239-1312.
- Gottsater A. Managing risk factors for atherosclerosis in critical limb ischaemia. Eur J Vasc Endovasc Surg. 2006; 32(5): 478-483.
- Zeller T, Krankenberg H, Steinkamp H, Rastan A, Sixt S, Schmidt A, et al. One-year outcome of percutaneous rotational atherectomy with aspiration in infrainguinal peripheral arterial occlusive disease: the multicenter pathway PVD trial. J Endovasc Ther. 2009; 16(6): 653-662.
- Maehara A, Mintz GS, Shimshak TM, Ricotta JJ, 2nd, Ramaiah V, Foster MT, 3rd, et al. Intravascular ultrasound evaluation of JETSTREAM atherectomy removal of superficial calcium in peripheral arteries. EuroIntervention. 2015; 11(1): 96-103.











