Coronary Atherectomy, Part III of III: Orbital Atherectomy
 In this third article in the series, we present a case illustrating the clinical application of an atherectomy device, followed by a brief discussion. Part I (August 2016) focused on laser atherectomy and Part II (September 2016) focused on rotational atherectomy, both authored by Zaheed Tai, DO, FACC, FSCAI, Winter Haven Hospital, Winter Haven, Florida. In Part III, we focus on orbital atherectomy with Arthur Lee, MD, FACC, FSCAI.
In this third article in the series, we present a case illustrating the clinical application of an atherectomy device, followed by a brief discussion. Part I (August 2016) focused on laser atherectomy and Part II (September 2016) focused on rotational atherectomy, both authored by Zaheed Tai, DO, FACC, FSCAI, Winter Haven Hospital, Winter Haven, Florida. In Part III, we focus on orbital atherectomy with Arthur Lee, MD, FACC, FSCAI.
Case Report
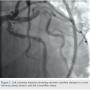
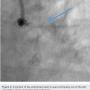
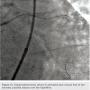
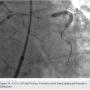
A 62-year-old man presented with chest pain and troponin leak. He had a history of end-stage renal disease on hemodialysis, critical limb ischemia status post left above-knee amputation, hypercholesterolemia, and ischemic colitis. He had also experienced a remote inferior infarct. His echo showed a newly decreased ejection fraction of 20%. Cardiac catheterization was performed, revealing a chronic total occlusion of the right coronary artery, and severe calcific obstructive disease in the left circumflex and ramus branch (Figure 1).
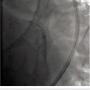
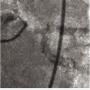
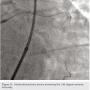
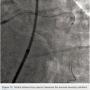
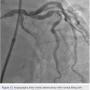
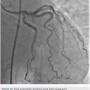
 The left circumflex was approached first and no vessel preparation was performed. A stent was not able to be delivered despite aggressive predilation and double wiring, which included rupturing several balloons (Figure 2). Upon withdrawing the stent after a failed delivery attempt, the stent was sheared off the balloon and dislodged in the proximal circumflex and into the left main artery (Figure 3). At that point, the decision was made by the operator to stent next to this first stent to pin it up against the wall of the circumflex, “excluding” it. This was done successfully (Figure 4).
The left circumflex was approached first and no vessel preparation was performed. A stent was not able to be delivered despite aggressive predilation and double wiring, which included rupturing several balloons (Figure 2). Upon withdrawing the stent after a failed delivery attempt, the stent was sheared off the balloon and dislodged in the proximal circumflex and into the left main artery (Figure 3). At that point, the decision was made by the operator to stent next to this first stent to pin it up against the wall of the circumflex, “excluding” it. This was done successfully (Figure 4).
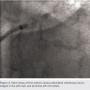
 However, the portion of the embolized stent in the left main was not recognized during this case and because of an acceptable balloon angioplasty result at the initial target lesion and a high radiation dose already administered, the procedure was terminated.
However, the portion of the embolized stent in the left main was not recognized during this case and because of an acceptable balloon angioplasty result at the initial target lesion and a high radiation dose already administered, the procedure was terminated.
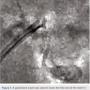
During follow-up film review in preparation to bring this patient back for staged intervention of the ramus branch, the left main portion of the embolized stent was recognized (Figure 5). Patient-related issues led to a delay of almost 2 weeks before returning to the lab (Figure 6).
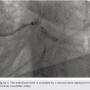
 Eventually, we embarked upon an effort to retrieve the “free” end of the stent in the left main. This was accomplished by using a gooseneck snare to grab the undeployed stent (Figure 7). Upon pulling the snared stent into the guide, the entire stent, including the portion that had been pinned up in the circumflex, was retrieved in one piece (Figure 8).
Eventually, we embarked upon an effort to retrieve the “free” end of the stent in the left main. This was accomplished by using a gooseneck snare to grab the undeployed stent (Figure 7). Upon pulling the snared stent into the guide, the entire stent, including the portion that had been pinned up in the circumflex, was retrieved in one piece (Figure 8).
Angiography showed no obvious injury to the vessels and after rewiring and post dilating the proximal circumflex stent, attention was then turned to the ramus branch. This vessel had a highly tortuous, severely calcified critical stenosis. Wiring with Frontline wires (Asahi Intecc) was initially unsuccessful. Eventually, a combination of an Asahi Fielder XT wire (Abbott Vascular) and a Corsair catheter (Asahi Intecc) was used to traverse this lesion (Figure 9).
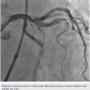
 Preparation of this vessel was completed with atherectomy in order to facilitate stent delivery and optimal stent expansion. The Diamondback 360 orbital atherectomy system (CSI) was chosen, given the severe tortuosity. With the orbital device, there is a leading edge bushing ahead of the “working area” of the device, which prevents the wire from being cut on tortuous turns. A 1.25 mm device was used and was delivered around the most extreme portion of the tortuous vessel where the critical lesion was located. Several runs on low and high were performed (Figures 10-11). We then dilated the lesion with a 3.0 mm balloon, and angiography showed adequate balloon expansion (Figure 13). A GuideLiner (Vascular Solutions) was placed prior to stent delivery to increase chances of stent delivery, but also to decrease the chance of stent embolization if the stent had to be withdrawn. A 4.0 x 28 mm Promus Premiere stent (Boston Scientific) was then delivered fairly easily with good stent expansion (Figure 14) and the procedure was finished safely (Figure 15).
Preparation of this vessel was completed with atherectomy in order to facilitate stent delivery and optimal stent expansion. The Diamondback 360 orbital atherectomy system (CSI) was chosen, given the severe tortuosity. With the orbital device, there is a leading edge bushing ahead of the “working area” of the device, which prevents the wire from being cut on tortuous turns. A 1.25 mm device was used and was delivered around the most extreme portion of the tortuous vessel where the critical lesion was located. Several runs on low and high were performed (Figures 10-11). We then dilated the lesion with a 3.0 mm balloon, and angiography showed adequate balloon expansion (Figure 13). A GuideLiner (Vascular Solutions) was placed prior to stent delivery to increase chances of stent delivery, but also to decrease the chance of stent embolization if the stent had to be withdrawn. A 4.0 x 28 mm Promus Premiere stent (Boston Scientific) was then delivered fairly easily with good stent expansion (Figure 14) and the procedure was finished safely (Figure 15).
Discussion
 This case highlights the fact that the lack of utilizing vessel preparation appropriately can result in lower procedural success rates, higher complication rates, higher contrast and radiation doses, and increased cost. It also demonstrates that orbital atherectomy can be performed safely in patients with severe left ventricular dysfunction and extremely tortuous vessels.
This case highlights the fact that the lack of utilizing vessel preparation appropriately can result in lower procedural success rates, higher complication rates, higher contrast and radiation doses, and increased cost. It also demonstrates that orbital atherectomy can be performed safely in patients with severe left ventricular dysfunction and extremely tortuous vessels.
Background
 CSI’s Diamondback Orbital Atherectomy System was approved in October of 2013 to treat severely calcified de novo coronary artery disease to facilitate stent implantation based on the results of the ORBIT II study. ORBIT II studied 443 coronary artery disease patients with severely calcified coronary lesions and demonstrated a 97.7% successful stent delivery with an impressive target lesion revascularization (TLR) rate of 3.4%, 5.1%, and 6.6% at 1, 2, and 3 years in the patients treated with orbital atherectomy and drug-eluting stent implantation (n=389/440).1–4 Complication rates were low and comparable to previous reported rates for the Rotablator. A recent real-world registry with 458 consecutive patients from 3 U.S. sites recently reported their experience.5 This registry included challenging patients with severely calcified coronary lesions, many of which would not have been included in the ORBIT II study, and confirmed the consistently low complication rates seen in the pivotal trial. Angiographic complication rates were perforation 0.7%, dissection 0.9%, and no-reflow 0.7%.
CSI’s Diamondback Orbital Atherectomy System was approved in October of 2013 to treat severely calcified de novo coronary artery disease to facilitate stent implantation based on the results of the ORBIT II study. ORBIT II studied 443 coronary artery disease patients with severely calcified coronary lesions and demonstrated a 97.7% successful stent delivery with an impressive target lesion revascularization (TLR) rate of 3.4%, 5.1%, and 6.6% at 1, 2, and 3 years in the patients treated with orbital atherectomy and drug-eluting stent implantation (n=389/440).1–4 Complication rates were low and comparable to previous reported rates for the Rotablator. A recent real-world registry with 458 consecutive patients from 3 U.S. sites recently reported their experience.5 This registry included challenging patients with severely calcified coronary lesions, many of which would not have been included in the ORBIT II study, and confirmed the consistently low complication rates seen in the pivotal trial. Angiographic complication rates were perforation 0.7%, dissection 0.9%, and no-reflow 0.7%.
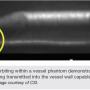
 The Diamondback orbital atherectomy system is shown in Figures 16-17. The orbital crown, which contains the band of embedded diamond grit, is eccentrically mounted on the shaft of the device. This results in a “wobble effect” when rotated about the shaft’s linear axis, which translates into an “orbit” of the crown around the axis of rotation (Figure 18). This orbiting action theoretically allows the working part of the crown to contact the vessel in a 360-degree fashion. The device also has two speeds, allowing for a variable radius of orbit; at the higher revolutions per minute (rpm) speed, the device will orbit at a higher radius.
The Diamondback orbital atherectomy system is shown in Figures 16-17. The orbital crown, which contains the band of embedded diamond grit, is eccentrically mounted on the shaft of the device. This results in a “wobble effect” when rotated about the shaft’s linear axis, which translates into an “orbit” of the crown around the axis of rotation (Figure 18). This orbiting action theoretically allows the working part of the crown to contact the vessel in a 360-degree fashion. The device also has two speeds, allowing for a variable radius of orbit; at the higher revolutions per minute (rpm) speed, the device will orbit at a higher radius.
 The orbit of the device allows more consistent and uniform flushing of the micro-particulate matter downstream and also allows the blood to constantly be flushing the area of contact, potentially limiting thermal injury, both of which are theoretical downsides of rotational atherectomy. There are also models showing an effect on deeper wall calcium that may be imparted by an outward “pulsatile force” exerted on the wall by a combination of the higher frequency rotation around the device axis, but more importantly, the lower frequency orbit around the same axis (Figure 19).
The orbit of the device allows more consistent and uniform flushing of the micro-particulate matter downstream and also allows the blood to constantly be flushing the area of contact, potentially limiting thermal injury, both of which are theoretical downsides of rotational atherectomy. There are also models showing an effect on deeper wall calcium that may be imparted by an outward “pulsatile force” exerted on the wall by a combination of the higher frequency rotation around the device axis, but more importantly, the lower frequency orbit around the same axis (Figure 19).
 The working portion of the nosecone is mounted approximately 5 mm behind the tip of the device (Figure 20). The leading edge bushing was a concern of ours when the device was commercially released, in that it might hamper device delivery to the most challenging lesions, but this has not been our experience. In fact, we now find that the short segment before the orbital crown to be an advantage. First, it helps to bring the orbital crown into tortuous lesions in a more coaxial path. The bushing tip also prevents the device from “cutting” its own wire, which has been reported with rotational atherectomy6-10, one of the reasons angulation greater than 45 degrees is a relative contraindication for rotational atherectomy.
The working portion of the nosecone is mounted approximately 5 mm behind the tip of the device (Figure 20). The leading edge bushing was a concern of ours when the device was commercially released, in that it might hamper device delivery to the most challenging lesions, but this has not been our experience. In fact, we now find that the short segment before the orbital crown to be an advantage. First, it helps to bring the orbital crown into tortuous lesions in a more coaxial path. The bushing tip also prevents the device from “cutting” its own wire, which has been reported with rotational atherectomy6-10, one of the reasons angulation greater than 45 degrees is a relative contraindication for rotational atherectomy.
However, severe wire bias can still be a challenge and therefore, an image of the wire and how it sits in the vessel should be taken prior to delivering the orbital atherectomy system, especially in a tortuous vessel where there can be pleating and significant wire bias.
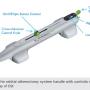
 There is one commercially approved coronary device, the 1.25 mm crown. It is similar to the 1.25 mm Classic crown that is still available for peripheral use, but the throw distance has been reduced, the medium speed setting has been eliminated, the tip has been optimized for better tracking in tortuous anatomy with a shorter bushing, and the wire is different as well. It must be used over the .014-inch coronary ViperWire. The ViperWire is a hydrophobic, soft-tip wire with 1.4 gram tip load. The ViperWire tapers transiently down to .007 inches before returning to an .012-inch diameter wire in the body. It is a fairly maneuverable, deliverable, and supportive wire, so much so that we routinely use this wire as our balloon and stent delivery wire after orbital atherectomy. The Diamondback is 6 French (Fr) compatible. It can also be delivered through a 6 Fr GuideLiner, but our preference is to place a GuideLiner after the orbital atherectomy system has been removed. It can be used radially as well. The handle has all the controls embedded in it, so there is no second pedal, including the two speed selectors, activation button, advancer lever, and a flush button to help deliver any vasodilators that can be delivered through the device. There are only two attachments to the handle, an electric cord to provide power and a flush line that is connected to an intravenous bag that supplies a lubricious flush mix called ViperSlide. The setup is extremely easy and quick.
There is one commercially approved coronary device, the 1.25 mm crown. It is similar to the 1.25 mm Classic crown that is still available for peripheral use, but the throw distance has been reduced, the medium speed setting has been eliminated, the tip has been optimized for better tracking in tortuous anatomy with a shorter bushing, and the wire is different as well. It must be used over the .014-inch coronary ViperWire. The ViperWire is a hydrophobic, soft-tip wire with 1.4 gram tip load. The ViperWire tapers transiently down to .007 inches before returning to an .012-inch diameter wire in the body. It is a fairly maneuverable, deliverable, and supportive wire, so much so that we routinely use this wire as our balloon and stent delivery wire after orbital atherectomy. The Diamondback is 6 French (Fr) compatible. It can also be delivered through a 6 Fr GuideLiner, but our preference is to place a GuideLiner after the orbital atherectomy system has been removed. It can be used radially as well. The handle has all the controls embedded in it, so there is no second pedal, including the two speed selectors, activation button, advancer lever, and a flush button to help deliver any vasodilators that can be delivered through the device. There are only two attachments to the handle, an electric cord to provide power and a flush line that is connected to an intravenous bag that supplies a lubricious flush mix called ViperSlide. The setup is extremely easy and quick.
Procedural Deployment
 A coaxial support guide is recommended. We recommend placing a temporary pacing wire for treatment of right coronary artery lesions, but operator practices will likely vary, as they do for rotational atherectomy in this situation. The lesion can be wired with the ViperWire, but depending on the complexity of the vessel and lesion, any long frontline wire can be used and then exchanged with a support catheter. A short frontline wire can be exchanged using a trapping balloon technique. We like to take an angiogram with the wire in place, especially in the right coronary and left circumflex, to see where there might be wire bias or pleating. These are areas where the device may be partially restricted in its orbit and demand special attention to minimize the risk of vessel perforation.
A coaxial support guide is recommended. We recommend placing a temporary pacing wire for treatment of right coronary artery lesions, but operator practices will likely vary, as they do for rotational atherectomy in this situation. The lesion can be wired with the ViperWire, but depending on the complexity of the vessel and lesion, any long frontline wire can be used and then exchanged with a support catheter. A short frontline wire can be exchanged using a trapping balloon technique. We like to take an angiogram with the wire in place, especially in the right coronary and left circumflex, to see where there might be wire bias or pleating. These are areas where the device may be partially restricted in its orbit and demand special attention to minimize the risk of vessel perforation.
 The device is then loaded on the wire. We recommend placing the advancer knob to the 1-2 cm mark prior to introduction in the guide, so the system can be backed up after final positioning to take off some of the stored forward torque acquired during advancement of the device. The tuohy valve should not be over-tightened on the shaft of the device. The orbital atherectomy device is advanced as a unit in an over-the-wire fashion to the lesion. In cases where the device cannot be advanced to the desired location, locking the wire brake down at that point and then advancing just the crown via the advancer knob will usually get you further to the lesion. The final motion prior to activating the device should be a reversal of the advancer knob to take off some of the stored forward momentum, so that the device does not jump forward during initial activation.
The device is then loaded on the wire. We recommend placing the advancer knob to the 1-2 cm mark prior to introduction in the guide, so the system can be backed up after final positioning to take off some of the stored forward torque acquired during advancement of the device. The tuohy valve should not be over-tightened on the shaft of the device. The orbital atherectomy device is advanced as a unit in an over-the-wire fashion to the lesion. In cases where the device cannot be advanced to the desired location, locking the wire brake down at that point and then advancing just the crown via the advancer knob will usually get you further to the lesion. The final motion prior to activating the device should be a reversal of the advancer knob to take off some of the stored forward momentum, so that the device does not jump forward during initial activation.
 When positioning the device, the leading tip of the device should be advanced into the lesion so that the tip is engaged in the lesion. This is particularly true for aorto-ostial and ostial circumflex lesions. This positioning will constrain the orbit slightly and prevent sudden restrictive changes in the orbit that could lead to a higher chance of complication. Proper technique includes slow advancement at 1 mm/second in both the forward and reverse directions, as the device works in both directions. We use a slow back-and-forth eraser motion for very tight lesions when making the initial pilot channel, as the cardiac motion and the slow recommended speed can make progress of the crown difficult to observe and it avoids sitting in one spot too long. Short runs, limited to 30 seconds or less with adequate rest periods between runs, and judicious use of vasodilators is recommended. The device activation should not be stopped within a tight lesion to avoid entrapment, but it can be deactivated both proximal and distal to the lesion.
When positioning the device, the leading tip of the device should be advanced into the lesion so that the tip is engaged in the lesion. This is particularly true for aorto-ostial and ostial circumflex lesions. This positioning will constrain the orbit slightly and prevent sudden restrictive changes in the orbit that could lead to a higher chance of complication. Proper technique includes slow advancement at 1 mm/second in both the forward and reverse directions, as the device works in both directions. We use a slow back-and-forth eraser motion for very tight lesions when making the initial pilot channel, as the cardiac motion and the slow recommended speed can make progress of the crown difficult to observe and it avoids sitting in one spot too long. Short runs, limited to 30 seconds or less with adequate rest periods between runs, and judicious use of vasodilators is recommended. The device activation should not be stopped within a tight lesion to avoid entrapment, but it can be deactivated both proximal and distal to the lesion.
 There is not an exact algorithm as to how many runs on low and high speed should be made for a specific lesion. It should be individualized to the situation, and depends on the overall disease and calcium burden, as well as the degree of angulation, wire bias, distal runoff, overall anatomy, and left ventricular function. In the ORBIT II trial, we generally followed a recommendation of 2 runs on low speed and 1 run on high, with a “run” meaning a full traversal of the device through the lesion and back again. It is important to remember that the lack of or resolution of longitudinal resistance does not necessarily mean the device is no longer affecting the vessel, as the orbit can still be exerting outward radial forces on superficial calcification and outward pulsatile forces on deeper calcium.
There is not an exact algorithm as to how many runs on low and high speed should be made for a specific lesion. It should be individualized to the situation, and depends on the overall disease and calcium burden, as well as the degree of angulation, wire bias, distal runoff, overall anatomy, and left ventricular function. In the ORBIT II trial, we generally followed a recommendation of 2 runs on low speed and 1 run on high, with a “run” meaning a full traversal of the device through the lesion and back again. It is important to remember that the lack of or resolution of longitudinal resistance does not necessarily mean the device is no longer affecting the vessel, as the orbit can still be exerting outward radial forces on superficial calcification and outward pulsatile forces on deeper calcium.
 The device is removed like any other over-the-wire device and does not require a Dynaglide option at this time. After removal, we have always used the ViperWire to deliver the balloon and stent. The tapering of the .014-inch wire tip down to .007 inches for a short segment is important to remember if the operator plans on using this wire to deliver a post-dilation balloon or imaging catheter after a stent has been deployed, as resistance can be encountered due to the small gap between the catheter/balloon and the smaller diameters of the ViperWire. Any resistance is easily overcome by replacing the ViperWire with a standard .014-inch wire, which should be simple at that point.
The device is removed like any other over-the-wire device and does not require a Dynaglide option at this time. After removal, we have always used the ViperWire to deliver the balloon and stent. The tapering of the .014-inch wire tip down to .007 inches for a short segment is important to remember if the operator plans on using this wire to deliver a post-dilation balloon or imaging catheter after a stent has been deployed, as resistance can be encountered due to the small gap between the catheter/balloon and the smaller diameters of the ViperWire. Any resistance is easily overcome by replacing the ViperWire with a standard .014-inch wire, which should be simple at that point.
 Dealing with any potential complications is beyond the scope of this article, but proper preparation and technique will minimize these rare occurrences. Perforations should be dealt with like any other perforation, with immediate balloon inflation to control the extravasation, and then longer ballooning or covered stent implantation if feasible. Slow flow can be dealt with using vasodilators. Device entrapment in the coronary arteries, to our knowledge, has not been reported for this device yet, but has been reported for rotational atherectomy.7 From our experience in the periphery, device entrapment could be dealt with in multiple ways, but potential therapies to address this hypothetical situation in the coronaries can include wiring the lesion next to the entrapped crown and trying to place a small balloon to help free the device. The device shaft can be cut, the saline sheath removed, and an .035-inch catheter with a hydrophilic tapered tip such as a NaviCross catheter (Terumo) could be advanced along the shaft of the device as close to the crown as possible to help provide counter-traction on the device during attempted removal.
Dealing with any potential complications is beyond the scope of this article, but proper preparation and technique will minimize these rare occurrences. Perforations should be dealt with like any other perforation, with immediate balloon inflation to control the extravasation, and then longer ballooning or covered stent implantation if feasible. Slow flow can be dealt with using vasodilators. Device entrapment in the coronary arteries, to our knowledge, has not been reported for this device yet, but has been reported for rotational atherectomy.7 From our experience in the periphery, device entrapment could be dealt with in multiple ways, but potential therapies to address this hypothetical situation in the coronaries can include wiring the lesion next to the entrapped crown and trying to place a small balloon to help free the device. The device shaft can be cut, the saline sheath removed, and an .035-inch catheter with a hydrophilic tapered tip such as a NaviCross catheter (Terumo) could be advanced along the shaft of the device as close to the crown as possible to help provide counter-traction on the device during attempted removal.
Conclusion
 Orbital atherectomy can be a useful adjunct in treating patients with severely calcified coronary artery disease. It can increase successful stent delivery and may improve long-term patency rates of drug-eluting stents in these challenging environments, as was observed in the ORBIT II trial. In our experience, orbital atherectomy makes challenging cases more predictable and safer. Vessel preparation will become an even more important concept as bioresorbable scaffolds have now been commercially approved. These scaffolds are bulkier devices and will be more challenging to deliver. They also perform better and safer when deployed with complete expansion of the scaffold. Both of these factors lend themselves to benefit from vessel preparation using orbital atherectomy.
Orbital atherectomy can be a useful adjunct in treating patients with severely calcified coronary artery disease. It can increase successful stent delivery and may improve long-term patency rates of drug-eluting stents in these challenging environments, as was observed in the ORBIT II trial. In our experience, orbital atherectomy makes challenging cases more predictable and safer. Vessel preparation will become an even more important concept as bioresorbable scaffolds have now been commercially approved. These scaffolds are bulkier devices and will be more challenging to deliver. They also perform better and safer when deployed with complete expansion of the scaffold. Both of these factors lend themselves to benefit from vessel preparation using orbital atherectomy.
 Orbital atherectomy advantages:
Orbital atherectomy advantages:
- Ease of use
- Will not cut its own wire in tortuous vessels
- One size crown for vessels 2-4 mm
- Orbit motion can partially counteract wire bias
 Only device approved for severely calcified disease
Only device approved for severely calcified disease- Studied in patients with severe left ventricular dysfunction
- Consistent and long-term data available
Contraindications:
References
- Chambers JW, Feldman RL, Himmelstein SI, et al. Pivotal trial to evaluate the safety and efficacy of the orbital atherectomy system in treating de novo, severely calcified coronary lesions (ORBIT II). JACC Cardiovasc Interv. 2014; 7: 510-518.
- Généreux P, Lee AC, Kim CY, et al. Orbital atherectomy for treating de novo severely calcified coronary narrowing (1-year results from the pivotal ORBIT II trial). Am J Cardiol. 2015; 115: 1685-1690.
- Généreux P, Bettinger N, Redfors B, et al. Two-year outcomes after treatment of severely calcified coronary lesions with the orbital atherectomy system and the impact of stent types: Insight from the ORBIT II trial. Catheter Cardiovasc Interv. 2016 Apr 16. doi: 10.1002/ccd.26554. [Epub ahead of print]
- Chambers J. Orbital atherectomy treatment of severely calcified coronary lesions: final 3-year results of the ORBIT II trial. Cardiovascular Research Technologies (CRT) Conference. Washington, D.C., 2016.
- Lee MS, Shlofmitz E, Kaplan B, et al. Real-world multicenter registry of patients with severe coronary artery calcification undergoing orbital atherectomy. J Interv Cardiol. 2016 Aug; 29(4): 357-362.
- Sulimov DS, Abdel-Wahab M, Toelg R, Kassner G, Geist V, Richardt G. Stuck rotablator: the nightmare of rotational atherectomy. EuroIntervention. 2013 Jun 22; 9(2): 251-258.
- Kanazawa T, Kadota K, Mitsudo K. Successful rescue of stuck rotablator burr entrapment using a Kiwami straight catheter. Catheter Cardiovasc Interv. 2015; 86: 942-945.
- Tanaka Y, Saito S. Successful retrieval of a firmly stuck rotablator burr by using a modified STAR technique. Catheter Cardiovasc Interv. 2016; 87: 749-756.
- Kosowski M, Zimoch W, Kübler P, et al. Percutaneous retrieval of a detached rotational atherectomy burr. Postepy Kardiol Interwencyjnej. 2013; 9(3): 301-303.
- Woodfield S, Lopez A, Heuser R. Fracture of coronary guidewire during rotational atherectomy with coronary perforation and tamponade. Cathet Cardiovasc Diagn. 1998 Jun;44(2):220-223.
Disclosure: Dr. Arthur Lee reports that he is a consultant/speaker for CSI, trains for Cook Medical, is on the advisory board for Boston Scientific, and is a speaker for Medtronic.
Dr. Arthur Lee can be contacted at leeivc@gmail.com.











