Complex PCI Using Transradial Access
Disclosure: Dr. Nishith Vayada reports no conflicts of interest regarding the content herein. Dr. Pancholy reports he is a technical consultant for the transradial product line for Terumo, and a speaker for Pfizer.
The authors can be contacted via Dr. Samir Pancholy at pancholys@gmail.com.
The following case is the fourth in a series of transradial-focused reports directed by section editor Dr. Samir Pancholy. This case series is supported by an educational grant from Medtronic.
History
This is a 64-year-old male with diabetes mellitus, longstanding history of hypertension, chronic kidney disease, and chronic obstructive lung disease on home oxygen therapy who presents with retrosternal chest 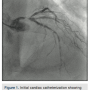 heaviness at rest, and severe resting shortness of breath. He was found to have pulmonary edema, with troponin I elevation (44 I.U), GFR=25ml/min, and hemoglobin of 9g/dl. Left ventricular ejection fraction (LVEF) by echocardiography was 25%, with severe anterior and lateral hypokinesia, moderate mitral regurgitation, and estimated right ventricular systolic pressure (RVSP)=55mmHg. Cardiac catheterization was performed and revealed a 90% distal eccentric calcified left main artery stenosis (Figure 1), tandem stenoses at the proximal, mid and distal left anterior descending artery (LAD), a 95% ostial calcified left circumflex stenosis, and severe diffuse disease in the proximal right coronary artery. No significant aortic stenosis was found. Pulmonary artery pressure was 70/30mmHg, with pulmonary capillary wedge pressure (PCWP)=20mmHg. Cardiac index, calculated using the Fick equation, was 1.9 l/min/m2.
heaviness at rest, and severe resting shortness of breath. He was found to have pulmonary edema, with troponin I elevation (44 I.U), GFR=25ml/min, and hemoglobin of 9g/dl. Left ventricular ejection fraction (LVEF) by echocardiography was 25%, with severe anterior and lateral hypokinesia, moderate mitral regurgitation, and estimated right ventricular systolic pressure (RVSP)=55mmHg. Cardiac catheterization was performed and revealed a 90% distal eccentric calcified left main artery stenosis (Figure 1), tandem stenoses at the proximal, mid and distal left anterior descending artery (LAD), a 95% ostial calcified left circumflex stenosis, and severe diffuse disease in the proximal right coronary artery. No significant aortic stenosis was found. Pulmonary artery pressure was 70/30mmHg, with pulmonary capillary wedge pressure (PCWP)=20mmHg. Cardiac index, calculated using the Fick equation, was 1.9 l/min/m2.
The patient was evaluated by 2 cardiothoracic surgeons, with a calculated Society of Thoracic Surgeons (STS) predicted mortality risk of 9.7%, and LAD being a poor target for bypass, the patient was deemed a poor candidate for coronary artery bypass graft surgery. After extensive discussion with the patient and the family, a high-risk percutaneous coronary intervention (PCI) was contemplated, using an intraprocedural left ventricular assist device. Using the CRUSADE bleed score, the patient’s estimated risk of in-hospital major bleeding was 19.5%. Bilateral ilio-femoral ultrasound revealed no iliac artery stenosis and adequate lumen for insertion of large-bore devices. An Impella (Abiomed) assisted rota-stenting of the unprotected left main coronary artery bifurcation was planned. The patient was pretreated with ticagrelor (Brilinta, AstraZeneca) in 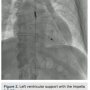 addition to aspirin, and contrast prep using isotonic saline was administered starting 12 hours before the procedure.
addition to aspirin, and contrast prep using isotonic saline was administered starting 12 hours before the procedure.
Procedure
A 6 French (Fr) hydrophilic introducer sheath was placed in the right radial artery in a standard fashion. A 14Fr introducer sheath with a 3.5 Impella CP catheter was placed via the right femoral artery using micropuncture access, into the left ventricular cavity and left ventricular (LV) support was initiated (Figure 2). A 5Fr pulmonary artery (PA) catheter was placed via the right basilic vein and right heart pressures were measured. Therapeutic doses of unfractionated heparin were administered and an Impella anticoagulation protocol was followed. 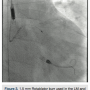
A 6Fr Extra Back Up (EBU) 4.0 guide catheter (Medtronic) was used to intubate the left main (LM) ostium, and an 0.014-inch, 180cm Runthrough NS guide wire (Terumo) was placed in the distal left circumflex (LCX). The guide wire was exchanged for an 0.014-inch, 325cm RotaWire Floppy guide wire (Boston Scientific) using a FineCross (Terumo) exchange catheter. A 1.5mm Rotablator burr (Boston Scientific) was used to perform 8 passes at 160,000 rpm with each pass <5 seconds in duration (Figure 3). The lesions at the distal LM and LCX ostium were successfully crossed. The guide wire was exchanged using a FineCross catheter, for a Runthrough NS wire. A second 0.014-inch, 180cm Runthrough NS guidewire was placed in the LAD. A 3.5 x 10mm Flextome Cutting Balloon (Boston Scientific) was used to dilate the LM-LCX junction. The same balloon was placed at the LM-LAD junction and that lesion was dilated. A 3Fr intravascular ultrasound (IVUS) catheter was placed and pre-stent IVUS indicated a reference lumen diameter of 3.6mm at the proximal LAD, and 4.3mm at the distal LM. A 3.5 x 24mm Resolute Integrity stent (Medtronic) was deployed at the proximal LCX into the LM at 20 atmospheres (atm). The 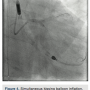 LAD guide wire was withdrawn and the LAD was rewired through the LM portion of the stent. A 3.5 x 15mm Sprinter balloon (Medtronic) was used to cross into the LAD and inflated at 18atm. A 3.5 x 12mm Resolute Integrity stent was deployed at the proximal LAD into the LM at 18atm. The LCX guide wire was pulled back into the guide catheter, and re-advanced into the LCX through the LM stent. Two 3.5 x 15mm NC Sprinter balloons were placed from the LM to the LCX and LAD, and a simultaneous “kissing” inflation was performed at 14atm (Figure 4). A 4.0 x 12mm NC Sprinter balloon was inflated in the LM portion of the stents at 18atm for proximal optimization. IVUS examination revealed excellent stent apposition (Figures 5a-5b). The stenoses in the proximal, mid and distal LAD were not treated. All catheters were removed except the Impella catheter, which was anchored in place using sutures.
LAD guide wire was withdrawn and the LAD was rewired through the LM portion of the stent. A 3.5 x 15mm Sprinter balloon (Medtronic) was used to cross into the LAD and inflated at 18atm. A 3.5 x 12mm Resolute Integrity stent was deployed at the proximal LAD into the LM at 18atm. The LCX guide wire was pulled back into the guide catheter, and re-advanced into the LCX through the LM stent. Two 3.5 x 15mm NC Sprinter balloons were placed from the LM to the LCX and LAD, and a simultaneous “kissing” inflation was performed at 14atm (Figure 4). A 4.0 x 12mm NC Sprinter balloon was inflated in the LM portion of the stents at 18atm for proximal optimization. IVUS examination revealed excellent stent apposition (Figures 5a-5b). The stenoses in the proximal, mid and distal LAD were not treated. All catheters were removed except the Impella catheter, which was anchored in place using sutures.
Hospital course
The patient arrived at the CCU in stable condition and had an uneventful initial 6 hours. Abrupt hypotension was noted, with a drop in cardiac index, PCWP and PA pressures. A significant increase in right thigh size was noted. Hemoglobin decreased to 6g/dl from 10g/dl immediately after the percutaneous coronary intervention (PCI) procedure. A right femoral bleed was suspected, and Impella support was rapidly weaned. 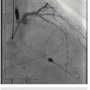 Vascular surgery was immediately consulted and the Impella catheter was removed. After 3 units of packed red blood cell transfusion, hemodynamics stabilized. The patient transitioned from the CCU to a monitored floor and after 5 days, to a rehabilitation unit where he recovered for 2 weeks and then was discharged to home.
Vascular surgery was immediately consulted and the Impella catheter was removed. After 3 units of packed red blood cell transfusion, hemodynamics stabilized. The patient transitioned from the CCU to a monitored floor and after 5 days, to a rehabilitation unit where he recovered for 2 weeks and then was discharged to home.
Follow-up
At 12-week follow-up, he remained asymptomatic, with an increase in LVEF to 45% by echocardiography. A pharmacologic myocardial perfusion study revealed a moderate sized, mild to moderate intensity mid to distal anterior perfusion abnormality with no reversibility. He continues to maintain an active life despite comorbidities with no angina or hospitalization for heart failure. 
Discussion
With the advent of second-generation drug-eluting stents and mechanical circulatory support with effective percutaneous applicability, high-risk PCI has become more available compared to previous years. As the patients who need these very high-risk interventions have a very high-risk substrate prone to complications, managing this risk to effectively mitigate these complications is of paramount importance. Patients presenting with high-risk ischemic syndromes are amongst the highest risk for bleeding complications.1 In addition to coronary and cardiac risk, which has decreased with the availability of advanced devices, bleeding risk continues to be a major post-procedural feature of these endeavors.
Transradial access typically has been considered applicable mostly in patients and lesion subsets with mild to moderate complexity. However, contrary to this common misconception, transradial access can be used in very complex subsets for PCI with equivalent success. Techniques frequently necessary in high-risk PCI such as rotational atherectomy2, ability to treat complex bifurcations3, embolic protection devices, thrombectomy, and intracoronary imaging equipment, can all be used with 6Fr access, and on occasion >6Fr access can be obtained using transradial access.
One of the major domains of bleeding risk in the complex patient cohort is access site bleeding. Decreasing the number of femoral arterial punctures might decrease access site bleeding risk and intensity. It has been demonstrated in a large international registry that patients requiring mechanical circulatory support during PCI experience a lower risk of complications, if the PCI portion of the procedure is performed using transradial access, compared to the strategy of using 2 femoral artery access sites.4 Hence, transradial access might not only be an equivalent, but a desirable, strategy in this very high-risk PCI subset, in order to lower the overall risk.
The other lesson from this case is the reminder that femoral access, albeit justifiable for large-bore access, always portends a risk of bleeding complications. Despite an uneventful procedure, with excellent stability clearly delivered by LV mechanical circulatory support in a very unfavorable baseline hemodynamic substrate, leaving large-bore devices in for longer durations, although reassuring, increases the risk, as evident in this instance. Systematic evaluation and identification of endpoints to guide the operator on optimal timing of device removal may help better utilize this major adjunct to high-risk PCI.
In summary, in procedures requiring LV mechanical circulatory support, high-risk PCI via transradial access is feasible, and might even be preferred over a “bi-femoral” approach. Femoral access, no matter how carefully obtained and cautiously attended, is prone to access site-related complications, especially in this morbid subset, and prompt recognition of these complications and definitive treatment helps mitigate worse consequences. A combination of the strategies proven to have the best impact on outcome improvement is expected to deliver a satisfactory long-term result. n
References
- Mehta SK, Frutkin AD, Lindsey JB, House JA, Spertus JA, Rao SV, Ou FS, Roe MT, Peterson ED, Marso SP; National Cardiovascular Data Registry. Bleeding in patients undergoing percutaneous coronary intervention: the development of a clinical risk algorithm from the National Cardiovascular Data Registry. Circ Cardiovasc Interv. 2009 Jun; 2(3): 222-229.
- Dahdouh Z, Roule V, Dugué AE, Sabatier R, Lognoné T, Grollier G. Rotational atherectomy for left main coronary artery disease in octogenarians: transradial approach in a tertiary center and literature review. J Interv Cardiol. 2013 Apr; 26(2): 173-182.
- Yang YJ1, Kandzari DE, Gao Z, Xu B, Chen JL, Qiao SB, Li JJ, Qin XW, Yao M, Wu YJ, Yuan JQ, Chen J, Liu HB, Dai J, Chen T, Wang Y, Li W, Gao RL. Transradial versus transfemoral method of percutaneous coronary revascularization for unprotected left main coronary artery disease: comparison of procedural and late-term outcomes. JACC Cardiovasc Interv. 2010 Oct; 3(10): 1035-1042.
- Romagnoli E, De Vita M, Burzotta F, Cortese B, Biondi-Zoccai G, Summaria F, Patrizi R, Lanzillo C, Lucci V, Cavazza C, Tarantino F, Sangiorgi GM, Lioy E, Crea F, Rao SV, Trani C. Radial versus femoral approach comparison in percutaneous coronary intervention with intraaortic balloon pump support: the RADIAL PUMP UP registry. Am Heart J. 2013 Dec; 166(6): 1019-1026.










