Chocolate CHIP* Intervention: An Orbital Experience
*Complex Higher-risk (and Indicated) Patients
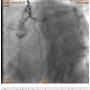
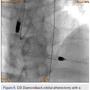
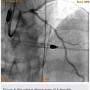
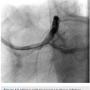
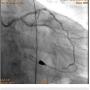
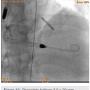
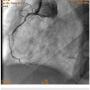 A 56-year-old man was on regular follow-up in our congestive heart failure (CHF) clinic for CHF with decreased ejection fraction. He gradually developed new Canadian Cardiovascular Society (CCS) III-IV anginal symptoms and had a cardiac catheterization revealing an angiographically significant, highly calcified proximal left anterior descending coronary artery (LAD) stenosis (90%) (Figure 1), intermediate left main (LM) disease (40-50%) (Figure 2), and complete occlusion of the distal right coronary artery (RCA) (Figure 3). Cardiac magnetic resonance imaging (MRI) revealed that the left ventricular apex and posterior walls might have limited viability.
A 56-year-old man was on regular follow-up in our congestive heart failure (CHF) clinic for CHF with decreased ejection fraction. He gradually developed new Canadian Cardiovascular Society (CCS) III-IV anginal symptoms and had a cardiac catheterization revealing an angiographically significant, highly calcified proximal left anterior descending coronary artery (LAD) stenosis (90%) (Figure 1), intermediate left main (LM) disease (40-50%) (Figure 2), and complete occlusion of the distal right coronary artery (RCA) (Figure 3). Cardiac magnetic resonance imaging (MRI) revealed that the left ventricular apex and posterior walls might have limited viability.

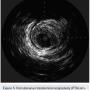
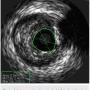 After undertaking initial medical optimization, the patient continued to have extensive symptoms with consumption of 5-6 nitroglycerin tablets (NTGs) on bad days and 2 NTGs on good days. Intravascular ultrasound (IVUS) of the LM was undertaken and this revealed a minimal lumen area (MLA) of 5.0 mm2, consistent with a significant LM stenosis (Figure 4). He was referred for coronary artery bypass grafting, but was felt to be a poor candidate due to his poor left ventricular (LV) systolic function. Therefore, we decided to undertake percutaneous coronary intervention (PCI) of his LAD and/or LM. The overall risk of complications of about 5-6% or so was discussed with patient, including the likely need for hemodynamic support.
After undertaking initial medical optimization, the patient continued to have extensive symptoms with consumption of 5-6 nitroglycerin tablets (NTGs) on bad days and 2 NTGs on good days. Intravascular ultrasound (IVUS) of the LM was undertaken and this revealed a minimal lumen area (MLA) of 5.0 mm2, consistent with a significant LM stenosis (Figure 4). He was referred for coronary artery bypass grafting, but was felt to be a poor candidate due to his poor left ventricular (LV) systolic function. Therefore, we decided to undertake percutaneous coronary intervention (PCI) of his LAD and/or LM. The overall risk of complications of about 5-6% or so was discussed with patient, including the likely need for hemodynamic support.
During this procedure attempt, left common femoral access was obtained using micropuncture technique, and angiography revealed a heavily calcified left external iliac artery stenosis and intermediate lesions in the right external and common iliac arteries. We were unable to place anything larger than a 10 French dilator through the left external iliac lesion without significant lower extremity and pelvic discomfort. Given significant patient discomfort and an inability to progress with the procedure, we decided to abort the procedure and reevaluate our options.
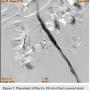
 In view of the increased complexity of this situation and potentially prolonged procedure times with increased risk of complications, we chose to stage the procedure in two phases: peripheral and coronary. Accordingly, the patient was brought in, the left common femoral artery was accessed using a micropuncture technique, and a 10 cm, 7 French sheath was placed in the artery. Angiography of the left iliofemoral system was performed using a 4 French multipurpose catheter. This was followed by IVUS, revealing a 38.1 mm2 reference segment area and a minimum 8.6 mm2 stenosis area, equating to an 80% area stenosis within the left external iliac artery (Figures 5-6). The wire was exchanged for a 260 cm Rosen wire (Cook Medical) and the lesion was first predilated with a Workhorse II 6 mm balloon (AngioDynamics) at 10 atmospheres for 60 seconds for two successive inflations. The balloon was then removed over the wire and the short 7 French sheath was exchanged over the wire for a 25 cm, 7 French sheath, which was advanced over the Rosen wire into the distal aorta. An 8 mm x 38 mm x 120 mm iCast covered stent (Atrium Medical) was then advanced to the level of the stenosis and unsheathed via retraction of the 7 French long sheath. The stent was deployed at 10 atmospheres for 60 seconds. There was still a small waist in the proximal stent and this was post dilated with the stent balloon at 13 atmospheres for 90 seconds with excellent angiographic result and no evident perforation or dissection, yielding 0% residual stenosis (Figure 7).
In view of the increased complexity of this situation and potentially prolonged procedure times with increased risk of complications, we chose to stage the procedure in two phases: peripheral and coronary. Accordingly, the patient was brought in, the left common femoral artery was accessed using a micropuncture technique, and a 10 cm, 7 French sheath was placed in the artery. Angiography of the left iliofemoral system was performed using a 4 French multipurpose catheter. This was followed by IVUS, revealing a 38.1 mm2 reference segment area and a minimum 8.6 mm2 stenosis area, equating to an 80% area stenosis within the left external iliac artery (Figures 5-6). The wire was exchanged for a 260 cm Rosen wire (Cook Medical) and the lesion was first predilated with a Workhorse II 6 mm balloon (AngioDynamics) at 10 atmospheres for 60 seconds for two successive inflations. The balloon was then removed over the wire and the short 7 French sheath was exchanged over the wire for a 25 cm, 7 French sheath, which was advanced over the Rosen wire into the distal aorta. An 8 mm x 38 mm x 120 mm iCast covered stent (Atrium Medical) was then advanced to the level of the stenosis and unsheathed via retraction of the 7 French long sheath. The stent was deployed at 10 atmospheres for 60 seconds. There was still a small waist in the proximal stent and this was post dilated with the stent balloon at 13 atmospheres for 90 seconds with excellent angiographic result and no evident perforation or dissection, yielding 0% residual stenosis (Figure 7).
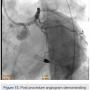
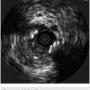
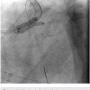 To further characterize the right common femoral artery (CFA) and common iliac artery (CIA) stenoses, the contralateral common iliac was engaged using an Omniflush catheter (AngioDynamics) and although the wire was easily advanced into the right CFA, the Omniflush would not advance further into the common iliac artery due to tortuosity. Therefore, a 4 French multipurpose angiographic (MPA) catheter was placed in the proximal right common iliac artery and a 0.014-inch pressure wire (Volcano Corp.) was used to measure simultaneous translesional pressure gradients distally and via pullback, revealing a total gradient of no more than 9 mmHg, indicating no significant flow limitation.
To further characterize the right common femoral artery (CFA) and common iliac artery (CIA) stenoses, the contralateral common iliac was engaged using an Omniflush catheter (AngioDynamics) and although the wire was easily advanced into the right CFA, the Omniflush would not advance further into the common iliac artery due to tortuosity. Therefore, a 4 French multipurpose angiographic (MPA) catheter was placed in the proximal right common iliac artery and a 0.014-inch pressure wire (Volcano Corp.) was used to measure simultaneous translesional pressure gradients distally and via pullback, revealing a total gradient of no more than 9 mmHg, indicating no significant flow limitation.
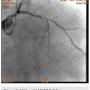
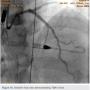 Following successful completion of the peripheral phase, it was felt that a 4-week period was necessary for the iliac stent to heal somewhat (especially since we planned to place a 14 French Cook sheath through it). We then moved on to the coronary phase of this procedure. Bilateral groins and the right wrist were prepared and draped in the usual sterile fashion. Using micropuncture technique with fluoroscopic verification of needle entry into the left CFA at the upper quadrant of the femoral head, a 6 French sheath was placed in the left CFA. The existing left femoral arterial sheath was removed, and the arteriotomy was pre-closed with a single Perclose device (Abbott Vascular). The arteriotomy was then serially dilated with 10, 12 and 14 French dilators, and a 14 French, 30 cm Cook sheath was placed successfully in the left common femoral artery. Following therapeutic anticoagulation with intravenous heparin, a regular J-wire and a 6 French pigtail catheter were used to cross the aortic valve, and the catheter was used to exchange the J wire for the 0.018-inch Platinum Plus wire (Boston Scientific). The Impella CP (3.5) percutaneous left ventricular assist device (LVAD) (Abiomed) was inserted into the left ventricle over the Platinum Plus wire (Figure 8). The Impella was then activated and good flows were verified. Right radial artery access was obtained and a PB 3.0 7.5 French sheathless guide system (Asahi Intecc) was used to engage the left main coronary artery. The LAD was wired uneventfully using a Runthrough wire (Terumo) via a 1.25 x 6 mm over-the-wire (OTW) Sprinter balloon (Medtronic). This was then exchanged for a Viper wire (CSI). Orbital atherectomy of the proximal LAD lesion was performed with excellent results (80,000 rpm x 4 passes, 120,000 rpm x 2 passes) (Figure 9). The LAD wire was then exchanged for a Runthrough wire and the left circumflex (LCX) was wired with a BMW wire. Following plaque modification with orbital atherectomy, predilation of LAD was performed with 3.0 x 20 mm Chocolate Balloon (QT Vascular Ltd) (15 atmospheres x 60 seconds and 15 atmospheres x 30 seconds) (Figure 10) with an excellent result and no dissection (Figure 11). A 3.5 x 38 mm Promus stent (Boston Scientific) (14 atmospheres x 30 seconds) was placed from the left main into the proximal LAD lesion ensuring adequate coverage. The jailed LCX wire was recovered and re-placed in the LCX through the struts of the newly placed LM-LAD stent (Figure 12). Predilation of the LCX ostium was performed with a 2.5 x 8 mm Glider balloon (QT Vascular Ltd) (14 atmospheres x 25 seconds). The mid LAD was stented (ensuring overlap with proximal stent) with 3.0 x 16 mm Promus stent (16 atmospheres x 25 seconds). A final kissing balloon inflation of the LAD/LCX with an NC Sprinter 2.5 x15 mm balloon (Medtronic) into the LCX and a NC Sprinter 3.5 x12 mm balloon into the LAD was performed, with up to 16 atmospheres x 20 seconds on both (Figure 13). Final IVUS of the LM and LAD stent revealed excellent results and the LCX ostium looked excellent as well (Figures 14-16).
Following successful completion of the peripheral phase, it was felt that a 4-week period was necessary for the iliac stent to heal somewhat (especially since we planned to place a 14 French Cook sheath through it). We then moved on to the coronary phase of this procedure. Bilateral groins and the right wrist were prepared and draped in the usual sterile fashion. Using micropuncture technique with fluoroscopic verification of needle entry into the left CFA at the upper quadrant of the femoral head, a 6 French sheath was placed in the left CFA. The existing left femoral arterial sheath was removed, and the arteriotomy was pre-closed with a single Perclose device (Abbott Vascular). The arteriotomy was then serially dilated with 10, 12 and 14 French dilators, and a 14 French, 30 cm Cook sheath was placed successfully in the left common femoral artery. Following therapeutic anticoagulation with intravenous heparin, a regular J-wire and a 6 French pigtail catheter were used to cross the aortic valve, and the catheter was used to exchange the J wire for the 0.018-inch Platinum Plus wire (Boston Scientific). The Impella CP (3.5) percutaneous left ventricular assist device (LVAD) (Abiomed) was inserted into the left ventricle over the Platinum Plus wire (Figure 8). The Impella was then activated and good flows were verified. Right radial artery access was obtained and a PB 3.0 7.5 French sheathless guide system (Asahi Intecc) was used to engage the left main coronary artery. The LAD was wired uneventfully using a Runthrough wire (Terumo) via a 1.25 x 6 mm over-the-wire (OTW) Sprinter balloon (Medtronic). This was then exchanged for a Viper wire (CSI). Orbital atherectomy of the proximal LAD lesion was performed with excellent results (80,000 rpm x 4 passes, 120,000 rpm x 2 passes) (Figure 9). The LAD wire was then exchanged for a Runthrough wire and the left circumflex (LCX) was wired with a BMW wire. Following plaque modification with orbital atherectomy, predilation of LAD was performed with 3.0 x 20 mm Chocolate Balloon (QT Vascular Ltd) (15 atmospheres x 60 seconds and 15 atmospheres x 30 seconds) (Figure 10) with an excellent result and no dissection (Figure 11). A 3.5 x 38 mm Promus stent (Boston Scientific) (14 atmospheres x 30 seconds) was placed from the left main into the proximal LAD lesion ensuring adequate coverage. The jailed LCX wire was recovered and re-placed in the LCX through the struts of the newly placed LM-LAD stent (Figure 12). Predilation of the LCX ostium was performed with a 2.5 x 8 mm Glider balloon (QT Vascular Ltd) (14 atmospheres x 25 seconds). The mid LAD was stented (ensuring overlap with proximal stent) with 3.0 x 16 mm Promus stent (16 atmospheres x 25 seconds). A final kissing balloon inflation of the LAD/LCX with an NC Sprinter 2.5 x15 mm balloon (Medtronic) into the LCX and a NC Sprinter 3.5 x12 mm balloon into the LAD was performed, with up to 16 atmospheres x 20 seconds on both (Figure 13). Final IVUS of the LM and LAD stent revealed excellent results and the LCX ostium looked excellent as well (Figures 14-16).
At the termination of the case, the Impella device was subsequently removed with no complications. A TR Band (Terumo) was placed over the radial arteriotomy, and the patient was discharged back to his room.
Multiple innovative strategies and techniques were in place to ensure the success of this procedure:
1. Patient selection
Appropriate patient selection is essential for the success of any procedure. Adequate evaluation was undertaken to ensure that the patient would benefit from the procedure with minimal risk. A heart team approach was undertaken to ensure that risks and benefits of alternative treatments and surgeries were considered.
2. Staging
For a complex procedure requiring difficult peripheral and coronary techniques, staging can be feasibly performed as long as the patient remains stable.
3. Access
The availability of the Asahi Intecc sheathless radial guide allows for a 7.5 French inner diameter guide catheter with a 5 French sheath outer diameter. This allows for complex procedures to be performed with excellent guide support.
4. Hemodynamic support
In patients in decreased LV function, in our experience, adequate hemodynamic support with a percutaneous LVAD goes a long way to enable success and promote safety.
5. Plaque modification equipment
In our experience, there are significant synergies to be exploited between the CSI Diamondback atherectomy device and the Chocolate balloon (QT Vascular Ltd). The orbital atherectomy device works by shaving or sanding calcified areas of the artery and may sometimes leave calcified spicules in the vessel that can be optimally modified further with adjunctive Chocolate balloon angioplasty, thus enabling excellent stent expansion and apposition, allowing for decreased restenosis and thrombosis. The Chocolate balloon consists of a mounted nitinol constraining structure specifically designed for uniform, controlled inflation and rapid deflation, resulting in atraumatic dilatation without the need for cutting or scoring. Because of its novel design, it is able to enable focused force angioplasty from its “pillows” while allowing plaque channeling through its “grooves”.
Disclosures: Orlando Marrero reports he is a consultant for Boston Scientific. Dr. Rupak Desai reports no conflicts of interest regarding the content herein. Dr. Gautam Kumar reports one consulting event with Abiomed in the last 12 months and one consulting event with Cardinal Health in the last 12 months.
Orlando Marrero, RCIS, MBA, can be contacted at orlm8597@icloud.com. Dr. Gautam Kumar can be contacted at gautam.kumar@emory.edu.