Can Upper Torso Vibro Acoustic Stimulation Treat No Reflow Following STEMI-Directed PPCI? Rationale and Literature Review. What’s the Buzzzzzzz?
Introduction
No-reflow following primary percutaneous coronary intervention (PPCI) in ST-elevation myocardial infarction (STEMI) occurs in up to 40% of cases, carries a poor prognosis, and at present, there are no consistently reliable strategies for treating this condition. Low sonic vibroacoustic stimulation (VAS) has shown to enhance clot disruption and stimulate vasodilation. Moreover, transthoracic diastolic timed VAS (tdVAS) (50 Hz, 2 mm) has shown to enhance myocardial relaxation, improve left ventricular (LV) output, and augment coronary flow in volunteers including those with coronary artery disease (CAD), all factors which may assist the no-reflow patient. We are not aware of any substantial review or analysis that has yet been done to address whether tdVAS could enhance emptying of an ischemic microvascular region following STEMI-directed coronary stenting. We have therefore performed a literature review on relatable data and provided mechanisms in consideration of this hypothesis.
Background
PPCI is the preferred reperfusion strategy in STEMI; however, there are times when myocardial reperfusion (or more precisely, coronary microvascular flow) does not restore to its optimal level, even following successful stenting of an infarct artery. This condition, known as slow or “no reflow”, has plagued PPCI in 10 to 40% of STEMI cases1-4, and carries with it an unacceptable increased risk of morbidity and mortality2. No reflow results in part from micro-embolization of athero-thrombotic material following balloon inflations into the arterioles and microcirculation which causes additional necrosis, inflammation, and microvascular dysfunction.2,4 Present treatment/prevention options include delivery of a variety of pharmaceuticals, as well as employment of distal protection and thrombus aspiration techniques — all of which have shown, at best, limited to mixed results in promotion of normalized reflow and/or clinical benefit.2,5-9 Indeed, there remains no consistently efficacious strategy for preventing or treating no reflow2,3, so the search continues for therapeutic options.
Transcutaneous ultrasound has been studied to improve STEMI reperfusion. However, the PLUS trial (27 kHz)10 failed to show efficacy, probably due to the problematic shielding of ultrasound by lung (which covers a substantial portion of the coronaries and does not transmit ultrasound) and a relatively thick, attenuating, chest wall.11 Since then, the need for skilled ultrasonic targeting across an acoustic penetration window was deduced and high mechanical index echocardiographic imaging, with clot-disruptive intravenous (IV) micro bubbles has since surged as a preferred investigative strategy in promoting reflow (or treating no reflow) in STEMI cases before and immediately after PPCI.3,12-14 However, results of a recent study regarding this technique were confusing, with 3 of 6 patients experiencing unresolved no reflow following stenting in patients pre-treated with ultrasound (an admitted safety concern requiring discontinuation of the study*).15 Ultrasound, especially when delivered at higher than standard duty factor or intensity, harbors a propensity to increase platelet adhesion16, promote bleeding17,18, and damage blood vessels.19-24 Studies in epicardial coronary arteries have in particular showed that ultrasonic energy alters endothelial function at high power and disrupts nitric oxide (NO)-dependent vasodilation25, and most recently, promotes vasoconstriction.15 Moreover, challenging acoustic imaging windows make it difficult to ensure ultrasonic penetration to a culprit coronary vessel11, and this problem is magnified by a paucity of microbubbles expected to actually reach an occlusive thrombosis site where there is absent flow.26
All in all, evidence for damage to membrane proteins and/or cytoskeletal fibers, complete membrane disruption, and irreversible cellular damage (including rupture of vascular endothelial cells resulting in capillary hemorrhage)27, along with difficulties in transcutaneously targeting ultrasound (and delivering microbubbles) to vascular targets, has been gradually mounting to disqualify ultrasound as a practical emergency reperfusive candidate.
Our group has been working on utilizing endothelial membrane ability to directly transform low sonic frequency VibroAcoustic Stimulation (VAS) into mechanical energy at the subcellular and cellular level. Unlike ultrasound, VAS does not promote violent cavitational air voids and bubbles in the tissue, and overall, can avoid damaging micro-stresses and strains to the cells caused by higher frequency ultrasonic waves. VAS stimulation has a long history of safe use in vascular stimulation and vasodilation. It has been proposed for treatment of a wide range of blood flow disorders, including improving the speed and quality of reperfusion in STEMI.11,28-31 VAS has been shown in vitro and in vivo to provide reliable transmissibility from the upper torso to the heart32,33, a clot-dissipating effect28,30,31,34-37, improvements in circulation, including microcirculation38-40, and a localized vasodilatory response41-44, particularly effective in vessels with spasm45,46.
Transthoracic “diastolic timed” VAS (tdVAS), delivered in avoidance of the early- to mid-force generation period of LV systole, has, in particular, shown to provide an enhancement of left ventricular (LV) relaxation and coronary flow in CAD patients32,33,47, as well as a positive inotropic effect in ischemic heart failure48,49. VAS also provides unique internal transmission characteristics through bodily tissue, including arteries and the epi-myocardium50-52, which suggests a less imperative need for perfect upper torso device positioning and skill-based targeting to ensure coronary stimulation11.
Potentially treatable components of no reflow include microcirculatory spasm, platelet aggregation, and micro-vascular thrombus53, which are all at least mechanistically treatable by tdVAS. Moreover, we have found no evidence that acute short-term exposures of VAS in the sonic frequency range poses harm to blood vessels**.
A review of the data
tdVAS augments coronary flow in volunteers with CAD
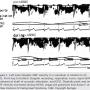 The response of coronary blood flow (CBF) velocity as measured by left main transesophageal Doppler in 10 healthy volunteers (8 men, 2 women; 50.6 ± 12.8 years old, mean ± SD) and 10 CAD patients (8 men, 2 women; 66.4 ± 8.0 years old) was compared immediately before, during and after tdVAS† (50 Hz, 2 mm amplitude) applied to the patient’s precordium.32 CBF velocity was also assessed by intracoronary Doppler flow wire in three CAD patients (2 men, 1 woman; 60.3 ± 10.5 years old). tdVAS was demonstrated to reliably transmit to the ventricle by observance of fluctuating perturbations in the LV pressure wave form and by transesophageal accelerometer. tdVAS was shown to immediately cause a statistically significant increase in LV (dP/dt) relaxation rate and diastolic CBF velocity (with no change in systolic flow, and independent from changes in heart rate or blood pressure) in all patients (Figure 1), with the most pronounced changes in CAD patients. The investigators concluded that tdVAS increased coronary flow by enhancing diastolic relaxation, likely by facilitated myocardial cross-bridge detachment in ischemic regions. The procedure was reported as safe, without any complications.
The response of coronary blood flow (CBF) velocity as measured by left main transesophageal Doppler in 10 healthy volunteers (8 men, 2 women; 50.6 ± 12.8 years old, mean ± SD) and 10 CAD patients (8 men, 2 women; 66.4 ± 8.0 years old) was compared immediately before, during and after tdVAS† (50 Hz, 2 mm amplitude) applied to the patient’s precordium.32 CBF velocity was also assessed by intracoronary Doppler flow wire in three CAD patients (2 men, 1 woman; 60.3 ± 10.5 years old). tdVAS was demonstrated to reliably transmit to the ventricle by observance of fluctuating perturbations in the LV pressure wave form and by transesophageal accelerometer. tdVAS was shown to immediately cause a statistically significant increase in LV (dP/dt) relaxation rate and diastolic CBF velocity (with no change in systolic flow, and independent from changes in heart rate or blood pressure) in all patients (Figure 1), with the most pronounced changes in CAD patients. The investigators concluded that tdVAS increased coronary flow by enhancing diastolic relaxation, likely by facilitated myocardial cross-bridge detachment in ischemic regions. The procedure was reported as safe, without any complications.
Diastolic timed VAS and positive inotropy
 The effects of diastolic timed VAS (in this case applied directly to the epi-myocardial surface, 50 Hz, 1 mm) in an open chest canine model of ischemic impaired LV relaxation was assessed.49 In 10 preparations, pacing tachycardia and administration of propranolol were imposed to induce various levels of incomplete relaxation. A flow probe was placed around the ascending aorta to measure stroke volume (SV). At resting heart rates, diastolic VAS caused an immediate decrease in the time constant (negative dP/dt) of LV pressure fall (T) without any influence on heart rate, LV peak systolic pressure (peak LVP), SV, and total systemic vascular resistance. With pacing tachycardia, diastolic VAS increased both peak LVP and SV at 160 beats per minute (before) and 120 beats per minute (after propranolol), simultaneously decreasing both T and LV diastolic pressures. The increase in peak LVP and SV caused by diastolic VAS correlated with the T diastolic interval (r=0.82), the assumed index of severity of incomplete relaxation. The investigators concluded that diastolic timed VAS accelerates LV relaxation rate and that this improves systolic function through the Frank-Starling mechanism. Figure 2 shows how diastolic timed VAS affected LV pressure in this study.
The effects of diastolic timed VAS (in this case applied directly to the epi-myocardial surface, 50 Hz, 1 mm) in an open chest canine model of ischemic impaired LV relaxation was assessed.49 In 10 preparations, pacing tachycardia and administration of propranolol were imposed to induce various levels of incomplete relaxation. A flow probe was placed around the ascending aorta to measure stroke volume (SV). At resting heart rates, diastolic VAS caused an immediate decrease in the time constant (negative dP/dt) of LV pressure fall (T) without any influence on heart rate, LV peak systolic pressure (peak LVP), SV, and total systemic vascular resistance. With pacing tachycardia, diastolic VAS increased both peak LVP and SV at 160 beats per minute (before) and 120 beats per minute (after propranolol), simultaneously decreasing both T and LV diastolic pressures. The increase in peak LVP and SV caused by diastolic VAS correlated with the T diastolic interval (r=0.82), the assumed index of severity of incomplete relaxation. The investigators concluded that diastolic timed VAS accelerates LV relaxation rate and that this improves systolic function through the Frank-Starling mechanism. Figure 2 shows how diastolic timed VAS affected LV pressure in this study.
Systolic VAS and negative inotropy
Precordial VAS stimulation (100 Hz, 2 mm) applied throughout the cardiac cycle (including systole) was shown to induce a mild depression of LV function in volunteers with known ischemic heart disease.54 This was discussed as potentially caused by VAS’s interference of LV cross-bridge kinetics during systole, and may therefore lead to decreased systemic blood pressures and elevated LV diastolic pressures (which may provoke heart failure, and/or increased levels of angina), should the technique be used clinically.
While this is not an expected problem for “diastolic timed” emissions, potential negative inotropy is a valid safety concern (and would need to be monitored for) should tdVAS be applied to the ischemic heart in future clinical trials.
VAS promotes clot dissipation
 An in vitro study was performed31 to assess whether intermittent, 50 Hz VAS (VAS applied during diastolic-like pressure and halted during systolic-like pressure) applied for 20 minutes over a 4 cm attenuating barrier would provide a clot-dissipating effect in a clotted, fluid-filled soft-flow catheter (lumen sized to resemble an epicardial coronary artery).VAS was shown to provide a substantial clot disruptive effect in all samples versus controls (Figure 3).
An in vitro study was performed31 to assess whether intermittent, 50 Hz VAS (VAS applied during diastolic-like pressure and halted during systolic-like pressure) applied for 20 minutes over a 4 cm attenuating barrier would provide a clot-dissipating effect in a clotted, fluid-filled soft-flow catheter (lumen sized to resemble an epicardial coronary artery).VAS was shown to provide a substantial clot disruptive effect in all samples versus controls (Figure 3).
Clot dissipation by VAS is not surprising in view of the Trellis catheter (Medtronic) which uses oscillations in the 50 Hz range to disrupt deep vein thrombosis.35 The use of VAS has also been shown to disrupt blood coagula endoscopically37 and external tapping of acutely thrombosed coronary arteries in open animal models have shown to immediately restore flow36. We also published a study showing how externally delivered 50 Hz VAS causes fluid turbulence which leads to clot erosion30, and have contributed to other studies showing how 24 Hz and 100 Hz VAS delivered, even remote from a thrombosis site, assists clearance of clotted flow systems34,28.
VAS enhances blood flow
It has been well established that low sonic VAS enhances regional circulation (including micro-circulation)38-40 and carries potent vasodilatory capabilities, particularly for arteries in spasm45,46. Cyclic stress and strain exerted on the endothelial lining of arteries can cause endogenous liberation of nitric oxide (NO), a potent vasodilator42-44, and tissue plasmin activator55, both factors predictive for stimulating regional blood flow.
In recent studies at Technion-Israel Institute of Technology (Haifa, Israel), it was shown that “sonophore” effect is responsible for bilayer membrane oscillation and translation of acoustic pressure wave into intracellular cyclic deformations, which in turn activates mechano-sensitive proteins.27
Researchers at Mt. Sinai Medical Center (Miami, Florida) have recently studied low sonic VAS to the chest wall of rats. This study showed enhanced endothelial NO release, which in addition to its vasodilatory properties, was discussed as a potential cardioprotective mechanism in limitation of ischemic reperfusion injury.41
Discussion/Conclusion
tdVAS may promote myocardial microvascular reflow following PPCI in STEMI cases. In view of favorable correlative data, pilot testing in no reflow appears compassionately warranted.
In Part 2 to this work, the authors will unveil a pilot protocol in use of a diastolic timed vibro-acoustic stimulator (Parallel Biotechnologies) to enable assessment of this technique.
References
- Soeda T, Higuma T, Abe N, Yamada M, Yokoyama H, Shibutani S, et al. Morphological predictors for no reflow phenomenon after primary percutaneous coronary intervention in patients with ST-segment elevation myocardial infarction caused by plaque rupture. Eur Heart J Cardiovasc Imaging. 2016 Jan 22. pii: jev341. [Epub ahead of print]
- Lim SY. No-reflow phoenomenon by intracoronary thrombus in acute Myocardial infarction. Chonnam Med J. 2016 Jan; 52(1): 38-44.
- Pacella J, Brands J, Schnatz FG, Black JJ, Chen X, Villanueva FS. Treatment of microvascular micro-embolization using microbubbles and long-tone-burst ultrasound: an in vivo study. Ultrasound Med Biol. 2015 Feb; 41(2): 456-464.
- Galasso G, Schiekofer S, D’Anna C, Gioia GD, Piccolo R, Niglio T, et al. No-reflow phenomenon: pathophysiology, diagnosis, prevention, and treatment. A review of the current literature and future perspectives. Angiology. 2014 Mar; 65(3): 180-189.
- Bavry AA, Kumbhani DJ, Bhatt DL. Role of adjunctive thrombectomy and embolic protection devices in acute myocardial infarction: a comprehensive meta-analysis of randomized trials. Eur Heart J. 2008; 29: 2989-2300.
- Svilaas T, Vlaar PJ, van der Horst IC, Diercks GF, de Smet BJ, van den Heuvel AF, et al. Thrombus aspiration during primary percutaneous coronary intervention. N Engl J Med. 2008; 358: 557-567.
- Stone GW, Webb J, Cox DA, Brodie BR, Qureshi M, Kalynych A, et al; Enhanced Myocardial Efficacy and Recovery by Aspiration of Liberated Debris (EMERALD) Investigators. Distal microcirculatory protection during percutaneous coronary intervention in acute ST-segment elevation myocardial infarction: a randomized controlled trial. JAMA. 2005 Mar 2; 293(9): 1063-1072.
- Gick M, Jander N, Bestehorn HP, Kienzle RP, Ferenc M, Werner K, et al. Randomized evaluation of the effects of filter-based distal protection on myocardial perfusion and infarct size after primary percutaneous catheter intervention in myocardial infarction with and without ST-segment elevation. Circulation. 2005; 112: 1462-1469.
- Ali A. Rheolytic thrombectomy in patients with acute MI did not result in a reduction in myocardial infarct size. Presented at Transcatheter Cardiovascular Therapeutics: 2004 September 27 to October 1; Washington, D.C.
- Hudson M, Greenbaum A, Brenton L, Gibson CM, Siegel R, Reeves LR, et al. Adjunctive transcutaneous ultrasound with thrombolysis: results of the PLUS (Perfusion by ThromboLytic and UltraSound) trial. JACC Cardiovasc Interv. 2010; 3(3): 352-359.
- Hoffmann A, Gill H. A study to determine chest wall vibratory attachment interface locations for a low frequency sonic vibrator in treatment of acute coronary thrombosis. J Thromb Thrombolysis. 2011 Aug; 32(2): 167-176.
- Wu J, Xie F, Lof J, Sayyed S, Porter TR. Utilization of modified diagnostic ultrasound and microbubbles to reduce myocardial infarct size. Heart. 2015 Sep; 101(18): 1468-74.
- Xie F, Wu J, Lof J, Sayyed S, Porter T. High mechanical index impulses from a diagnostic transducer during an intravenous microbubble infusion can reduce ultimate infarct size when compared to full dose tissue plasminogen activator in acute ST segment elevation myocardial infarction. J Am Coll Cardiol. 2014;63(12_S).
- Slikkerveer J, Kleijn SA, Appelman Y, Porter TR, Veen G, van Rossum AC, Kamp O. Ultrasound enhanced prehospital thrombolysis using microbubbles infusion in patients with acute ST elevation myocardial infarction: pilot of the Sonolysis study. Ultrasound Med Biol. 2012 Feb; 38(2): 247-252.
- Roos ST, Juffermans LJM, Van Royen N, Van Rossum AC, Xie F, Appelman Y, Porter TR, Kamp O. Unexpected high incidence of coronary vasoconstriction in the reduction of microvascular injury using Sonolysis (ROMIUS) trial. Ultrasound Med Biol. (Article in Press - Epub ahead of print), 5 May 2016. doi: https://dx.doi.org/10.1016/j.ultrasmedbio.2016.03.032
- Frizzel LA, Miller DL, Nyborg WL. Ultrasonically induced intravascular streaming and thrombus formation adjacent to a micropipette. Ultrasound Med Biol. 1986; 12(3): 217-221. doi: 10.1016/0301-5629(86)90312-1.
- Daffertshofer M, Gass A, Ringleb P, Sitzer M, Sliwka U, et al. Transcranial low-frequency ultrasound-mediated thrombolysis in brain ischemia: increased risk of hemorrhage with combined ultrasound and tissue plasminogen activator: results of a phase II clinical trial. Stroke. 2005; 36: 1441-1446.
- Eggers J, Koch B, Meyer K, Konig I, Seidel G. Effect of ultrasound on thrombolysis of middle cerebral artery occlusion. Ann Neurol. 2003 Jun; 53(6): 797-800.
- Kornowski R, Meltzer R. Does external ultrasound accelerate thrombolysis? Results from a rabbit model. Circulation. 1994; 89: 339-344.
- Mukherjee D, Wong J, Griffin B. Ten fold augmentation of endothelial uptake of vascular endothelial growth factor with ultrasound after systemic administration. J Am Coll Cardiol. 2000; 35(6): 1678-1686.
- Riggs PN, Francis CW, Bartos SR, Penney DP. Ultrasound enhancement of rabbit femoral artery thrombolysis. Cardiovasc Surg. 1997; 5(2): 201-207.
- Nishioka T, Luo H, Fishbein MC, et al. Dissolution of thrombotic arterial occlusion by high intensity, low frequency ultrasound and dodecafluoropentane emulsion: an in vitro and in vivo study. J Am Coll Cardiol. 1997; 30(2): 561-568.
- Luo H, Nishioka T, Fishbein MC. et al. Transcutaneous ultrasound augments lysis of arterial thrombi in vivo. Circulation. 1996; 94: 775-778.
- Kerr CL, Gregory DW, Chan KK, Wattmough DJ, Wheatly DN. Ultrasound-induced damage of veins in pig ears, as revealed by scanning electron microscopy. Ultrasound Med Biol. 1989; 15: 45-52.
- Discigil B, King RM, Pearson PJ, Capellini VK, Rodrigues AJ, Schaff Hartzell V, et al. High-frequency ultrasonic waves cause endothelial dysfunction on canine epicardial coronary arteries. Rev Bras Cir Cardiovasc. 2008 Apr-Jun;23(2):190-196.
- Xie F, Lof J, Everbach C, He A, Bennett RM, Matsunaga T, et al. Treatment of acute intravascular thrombi with diagnostic ultrasound and intravenous microbubbles. JACC Cardiovasc Imaging. 2009 Apr; 2(4): 511-518.
- Krasovitskia B, Frenkelb V, Shohama S, Kimmela E. Intramembrane cavitation as a unifying mechanism for ultrasound-induced bioeffects. Proc Natl Acad Sci U S A. 2011 Feb 22; 108(8): 3258-3263.
- Yohannes FG, Hoffmann AK: Non-invasive low frequency vibration as a potential adjunctive treatment for heart attack and stroke. An in-vitro flow model. J Thromb Thrombolysis. 2008 Jun; 25(3): 251-258.
- Hoffmann A, Gill H. The timing of onset of mechanical systole and diastole in reference to the QRS-T complex: a study to determine performance criteria for a non-invasive diastolic timed vibration massage system in treatment of potentially unstable cardiac disorders. Cardiovasc Eng. 2010 Dec; 10(4): 235-245.
- Hoffmann A, Gill H. Externally applied vibration at 50 Hz facilitates dissolution of blood clots in-vitro. Am J Biomed Sci. 2012; 4(4):274-284. doi: 10.5099/aj120400274
- Hoffmann A, Gill H. Diastolic timed vibro-percussion at 50 Hz delivered across a chest wall sized meat barrier enhances clot dissolution and remotely administered streptokinase effectiveness in an in-vitro model of acute coronary thrombosis, Thromb J. 2012; 10: 23. doi: 10.1186/1477-9560-10-23.
- Koiwa Y, Honda H, Naya T, Shirato K. Precordial or epicardial input of phase-controlled minute vibration: effect on the coronary flow rate in regional ischemia. In: New Horizons for Failing Heart Syndrome. Sasayama S, ed. Springer Japan: Tokyo, 1996:117-130.
- Koiwa Y, Honda H, Takagi T, Kikuchi J, Hoshi N, Takishima T. Modification of human left ventricular relaxation by small-amplitude, phase–controlled mechanical vibration on the chest wall. Circulation. 1997; 95: 156-162.
- Marzencki M, Kajbafzadeh B, Khosrow-khavar F, Tavakolian K, Soleimani-Nouri M, Hamburger J, et al. Low frequency mechanical actuation accelerates reperfusion in-vitro. Biomed Eng Pub Online. Nov 2013; 12: 121. doi: 10.1186/1475-925X-12-121.
- Arko F, Davis C, Murphy E, Smith S, Timaran C, Modrall G, et al. Aggressive percutaneous mechanical thrombectomy of deep venous thrombosis. Arch Surg. 2007; 142: 513-519. doi: 10.1001/archsurg.142.6513.
- Folts J. An in vivo model of experimental arterial stenosis, intimal damage, and periodic thrombosis. Circulation. 1991; 83: IV-3–IV-14.
- Wobser E, Stumpff U. Intragastral disintegration of blood coagula by mechanical vibration. Endoscopy. 1978; 10: 15-19. doi: 10.1055/s-0028-1098254.
- Nakagami G, Sanada H, Matsui N, Kitagawa A, Yokogawa H, Sekiya N, et al. Effect of vibration on skin blood flow in an in vivo microcirculatory model. BioScience Trends. 2007; 1(3): 161-166.
- Button C, Anderson N, Bradford C, Cotter JD, Ainslie PN. The effect of multidirectional mechanical vibration on peripheral circulation of humans. Clin Physiol Funct Imaging. 2007 Jul; 27(4): 211-216.
- Hudlicka O, Wright A. The effect of vibration on blood flow in skeletal muscle in rabbit. Clin Sci Mol Med. 1978, 55: 471-476.
- Aelen P, Uryash A, Woerlee P, Adams J. Abstract 201: in-vivo response of eNOS up-regulation by acoustic induced vibrations in rats. Circulation. 2010, 122: A201.
- Maloney-Hinds C, Petrofsky JS, Zimmerman G, Hessinger DA. The role of nitric oxide in skin blood flow increases due to vibration in healthy adults and adults with type 2 diabetes. Diabetes Technol Ther. 2009 Jan; 11(1): 39-43.
- Pei Z, Chen J, Zhu M, Liu J, Zhang Q. The effects of infrasound on the secretion of the nitric oxide in rat plasma and the expression of VEGF in vascular endothelia. Chinese Heart Journal. 2004. Available online at https://en.cnki.com.cn/Article_en/CJFDTOTAL-XGNZ200401005.htm. Accessed August 23, 2016.
- Weitzberg E, Lundberg J. Humming greatly increases nasal nitric oxide. Am J Respir Crit Care Med. 2002, 166: 144-145.
- Lindblad LE, Lorenz RR, Shepherd JT, Vanhoutte PM. Effect of vibration on canine cutaneous artery. Heart Circ Physiol. 1986; 19: H519-H523.
- Ljung B, Silvertsson R. Vibration-induced inhibition of vascular smooth muscle contraction. Blood Vessels. 1975, 12: 38-52.
- Naya T, Koiwa Y, Honda H. et al. Diastolic vibration from the precordium increases coronary blood flow in humans. J Cardiovasc Diagn Procedures. 1994; 12: 110. Abstract (FRI – POS07).
- Takagi T, Koiwa Y, Kikuchi J, et al. Diastolic vibration improves systolic function in cases of incomplete relaxation. Circulation. 1992; 86: 1955-1964.
- Koiwa Y, Takagi T, Kikuchi H, Honda H, Hoshi N, Takishima T. The improvement of systolic function of depressed left ventricle by external vibration at diastole. Tohoku J Exp Med. 1989; 159: 169-170.
- Farber JJ, Purvis JH. Conduction of cardiovascular sound along arteries. Circ Res. 1963; 12: 308-316.
- Hashiguchi R, Koiwa Y, Ohyama T, et al. Dependence of instantaneous transfer function on regional ischemic myocardial volume. Circ Res. 1988; 63: 1003-1011.
- Smith D, Ishimitsu T, Craige E. Mechanical vibration transmission characteristics of the left ventricle implication with regard to auscultation and phonocardiography. J Am Coll Cardiol. 1984; 4(3): 517-521.
- Kaul S. Sonothrombolysis: a universally applicable and better way to treat acute myocardial infarction and stroke? Who is going to fund the research? Circulation. 2009; 119: 1358-1360.
- Koiwa Y, Ohyama T, Takagi T, Takishima T. Clinical demonstration of vibration-induced depression of left ventricular function. Tohoku J Exp Med. 1989 Nov; 159(3): 247-248.
- Takashima N, Higashi T. Change in fibrinolytic activity as a parameter for assessing local mechanical stimulation during physical exercise. Eur J Appl Physiol Occup Physiol. 1994; 68(5): 445-449.
*See discussion of results regarding use of high mechanical index ultrasound with microbubbles pre and post cath in PPCI STEMI cases (the ROMIUS trial) by the following link: https://www.livemedia.gr/video/179544 (presentation by Professor Otto Kamp, from the 30th Annual Advances in Contrast Ultrasound - Bubble Conference 2015, Chicago).
**According to years of internet (including patent) searches by the authors, with search words including vibration, acoustics, percussion, hertz, sonic, infrasonic, artery, vein, thrombosis, coronary, cerebral, blood, blood flow, damage, endothelium, cavitation, bleeding, hemorrhage.
†VAS timed from late systole (i.e., peak contraction of the anterior wall by m-mode) to the following P wave.
1CEO, Parallel Biotechnologies LLC, Miami Beach, Florida; 2In-Vitro Laboratories, Burnaby, British Columbia, Canada; 3Ahof Biophysical Systems Inc., Burnaby, British Columbia, Canada.
Disclosures: This review was sponsored by a grant awarded by Ahof Biophysical Systems Inc., an entity holding patents relating to vibration for promoting coronary flow. Andrew Hoffmann holds shares in Ahof Biophysical Systems Inc. Material support was provided by Parallel Biotechnologies LLC. Arkady Uryash, MD, holds shares in Parallel Biotechnologies LLC, an entity also holding patents relating to vibration for treatment of blood flow disorders. Harjit Gill, PhD, has received funding from Ahof Biophysical Systems Inc. for this and other assignments.
Arkady Uryash, MD, can be contacted at auryashmd@parallelbiotech.com. Harjit Gill, PhD, can be contacted at h-gill7@hotmail.com. Andrew Hoffmann, BSc, can be contacted at andrew.hoffmann11@gmail.com.










